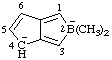 | 2,4-dihydro-2,2-dimethylcyclopenta[c]borol-4-id-2-uide |
Contents of this section
RC-83.4.1. Polyanions identically derived at different positions on the same parent hydride are named using appropriate numerical prefixes and operational suffixes as given in RC-83.1.2 and RC-83.2.
RC-83.4.2. "Ide" and "uide" centers in the same parent hydride. Anionic compounds with two or more anionic centers in the same parent hydride structure, at least one of which is derived formally by the removal of a hydron from a skeletal position, and one by the addition of a hydride ion at another skeletal position, are named by replacing the final "e" of the name of the parent hydride, if present, by the operational suffix "-ide", followed by the operational suffix "-uide", each preceded, where necessary, by the appropriate numerical or multiplicative prefix (see RC-83.1.2.1 and RC-83.2). The "e" of the "-ide" suffix is elided before "-uide" unless a multiplicative prefix precedes the latter. Where there is a choice, low locants of the parent hydride are assigned first to the anionic centers regardless of type and then to "-uide" anionic centers.
Example:
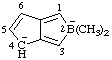 | 2,4-dihydro-2,2-dimethylcyclopenta[c]borol-4-id-2-uide |
Note: The multiplicative prefixes "bis-", "tris-", etc. are used to avoid the possibility of ambiguity which would result if a suffix such as "-diolate" were to be interpreted as describing a monoanion derived from a diol.Examples:
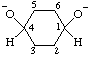 | cyclohexane-1,4-bis(olate) |
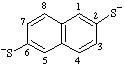 | naphthalene-2,6-bis(thiolate) |
Examples:
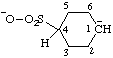 | cyclohexan-1-ide-4-sulfonate |
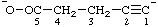 | pent-1-yn-1-id-5-oate |
Note: The multiplicative prefixes "bis-" and "tris-",. etc., are used to avoid the possibility for confusion that could arise from the use of the numerical prefixes "di-", "tri-", etc.Examples:
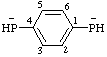 | 1,4-phenylenebis(phosphanide) |
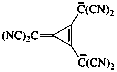 | tetracyano[3-dicyanomethylidene)cycloprop-1-ene-1,2-diyl]dimethanide (IUPAC multiplicative method; see RC-80.11) [3-(dicyanomethylidene)cycloprop-1-ene-1,2-diyl]bis[dicyanomethanide] (CAS multiplicative method; see RC-80.11) |
Note: Accordingly, anionic centers derived from two or more of such same characteristic groups are not described in terms of such characteristic groups using appropriate multiplicative prefixes.Examples:
| -O-O-CH2-CH2-O-O- | ethylenebis(dioxidanide) |
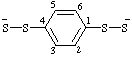 | 1,4-phenylenebis(disulfanide) |
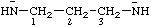 | propane-1,3-diylbis(amide) propane-1,3-diylbis(azanide) |
Example:
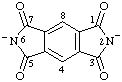 | 1,3,5,7-tetraoxo-1,2,3,5,6,7-hexahydrobenzo[1,2-c:4,5-c']dipyrrole-2,6-diide (see RC-83.1.2.2) 1,3,5,7-tetraoxo-5,7-dihydrobenzo[1,2-c:4,5-c']dipyrrole-2,6(1,3H)-diide (see RC-83.1.2.2) |
RC-83.4.7.1. Prefixes for anionic centers derived from acid characteristic groups by removal of the hydrogen atoms from all hydroxy, thiol, etc., groups of an acid characteristic group or a chalcogen analog, and that are attached to the parent structure by a single bond are named by changing the "-ic acid" ending of the name of the acid suffix or parent mononuclear oxo acid to "-ato" (ref 2vv, 4u).
Note: In the 1979 IUPAC Organic Rules, these prefixes (ref 4u) were not limited to attachment by a single bond.Examples:
| -CO-O- | carboxylato |
| -SO2-O- | sulfonato |
| -PO(O-)2 | phosphonato |
| -AsH(O)(O-) | arsinato |
Note: In the 1979 IUPAC Organic Rules, this prefix (ref 4u) was used for the anionic substituent group =As(O)-O-RC-83.4.7.2. Prefixes for anionic chalcogen atoms may be derived by replacing the final "e" of the name of the corresponding monoatomic anion (ref 6h) with an "o" (ref 2ww).
Example:
| -O- | oxido |
Examples:
| -CH2- | methanidyl |
| -NH2- | amidyl azanidyl |
| -N2- | imidyl azanediidyl |
| -PH- | phosphanidyl |
| -BH3- | boranuidyl |
| =N- | amidylidene (see RC-80.5) azanidylidene (see RC-80.5) azanidediyl (see RC-80.5) |
Note: The use of "-diyl" with the anion name "amide" is not recommended.
| -S-S- | disulfanidyl |
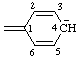 | cyclohexa-2,5-dien-4-id-1-ylidene (see RC-80.5) |
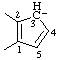 | cyclopenta-1,4-dien-3-ide-1,2-diyl (see RC-80.5) |
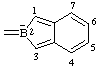 | 2H-benzo[c]borol-2-uid-2-ylidene (see RC-80.5) 2H-2-benzoborol-2-uid-2-ylidene (ref 3h) (see RC-80.5) |
(a) the maximum number of anionic centers of any kind, including anionic suffix groups;Note: This is in agreement with the principles for choosing a preferred component in naming fused heterocyclic ring systems (ref 3i).
(b) the maximum number of senior anionic centers, according to the nature of the anionic atoms, in the same order as the corresponding replacement prefixes (ref 3d, 11).Further choice, if necessary, is made by applying the general criteria for chains and rings given in the 1979 IUPAC Organic Rules (ref 2cc).
Examples:
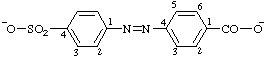 | 4-[(4-sulfonatophenyl)azo]benzoate |
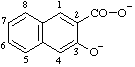 | 3-oxidonaphthalene-2-carboxylate |
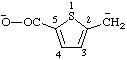 | (5-carboxylato-2-thienyl)methanide |
1. International Union of Pure and Applied Chemistry. Organic Chemistry Division. Commission on Nomenclature of Organic Chemistry, Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F and H, 1979 ed., Pergamon Press, Oxford, 1979, 559 p.
2. Reference 1, Section C, pp. 79-322: [q] Rules C-72, p. 130-1; and C-73, p. 132; [w] Rule C-106.2, p. 146; [cc] Subsections C-0.13, pp. 97-101; and C-0.14, pp. 101-5; [ww] Rule C-86.2, p. 142.
3. Reference 1, Section B, pp. 53-76: [d] Rule B-1.1, pp. 53-5, Table I, p. 53; [h] Rule B-3.5, pp. 67-8; [i] Rules B-6. pp. 71-2; B-10.1 (in part), p. 72; and B-11 (in part).
4. Reference 1, Section D, pp. 323-471: [u] Rule D-5.52, p. 396.
6. International Union of Pure and Applied Chemistry. Inorganic Chemistry Division. Commission on Nomenclature of Inorganic Chemistry, Nomenclature of Inorganic Chemistry, Recommendations 1990, G. J. Leigh, ed., Blackwell Scientific Publications, Oxford, England, 1990, 289 p. [h] Section I-8.3.2, p. 108.
11. International Union of Pure and Applied Chemistry. Organic Chemistry Division. Commission on Nomenclature of Organic Chemistry, Revision of the Extended Hantzsch-Widman System of Nomenclature for Heteromonocycles, Recommendations 1982, Pure Appl. Chem. 55, 409-16, 1983.