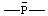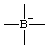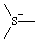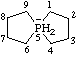Revised Nomenclature for Radicals, Ions, Radical Ions and Related Species
(IUPAC Recommendations 1993)
RC-83.2. Anionic Centers Derived Formally by the Addition of a Hydride Ion
Continued from Rule RC-83. Anions
Contents of this section
- RC-83.2. Anionic compounds with anionic centers derived formally by the addition of a hydride ion
- RC-83.3. Replacement nomenclature for anions
RC-83.2. Anionic compounds with anionic centers derived formally by the addition of a hydride ion. The nomenclature systems in the 1979 IUPAC Organic Rules (ref 1) do not provide an operational suffix for indicating addition of a hydride ion to a parent compound comparable to the "-ium" suffix for naming cations (see Rule RC-82.1.1.2). Although many such compounds can be named by the principles of coordination nomenclature (ref 6j), for example, tetrahydroborate (ref 6a), for [BH4]-, and hexachlorophosphate (ref 6k), for [PCl6]-, anions of this type occurring in more complicated organic systems cannot be named easily in this way. Therefore, two methods, other than replacement techniques (see RC-83.3), are included in these revised recommendations for use in naming such anions.
(a) The operational suffix "-uide". An anionic center at any position of a neutral parent hydride may be described by replacing the final "e" of the name of the parent hydride. if present, by the operational suffix "uide", or by adding the operational suffixes "-uide", "-diuide", etc., to the name of the parent hydride; locants for the anionic centers thus created are cited as needed or desired.
(b) The operational suffix "-ide" with the λ-convention (ref 16). An anionic center derived from any position of a neutral parent hydride where the λ-convention defines at least two more hydrogen atoms than are present in the standard neutral parent hydride may be described by replacing the final "e" of the parent hydride name, if present, by the operational suffix "-ide", or by adding the operational suffixes "-ide", "-diide". etc., to the name of the parent hydride. Locants for the anionic centers thus defined are cited, if necessary, even though locants are given with the "λ" symbolism.
Since method (b) often requires use of bonding states that may be too artificial for some to accept readily, method (a) is to be preferred in such cases; for example, this method would require [AsF6]- and [GeF5]- to be named hexafluoro-λ7-arsanide and pentafluoro-λ6-germanide, respectively.
Examples:
| CH3-SiH4- | (a) methylsilanuide |
| (CH3)4P- | (a) tetramethylphosphanuide (ref 4g, 6b)
(b) tetramethyl-λ5-phosphanide (ref 16)
(b) tetramethylphosphoranide (ref 4p) |
| (C6H5)F2S- | (a) difluoro(phenyl)sulfanuide
(b) difluoro(phenyl)-λ4-sulfanide (ref 16) |
| (C6H5)2I- | (a) diphenyliodanuide
(b) diphenyl-λ3-iodanide (ref 16) |
| F6I- | (a) hexafluoro-λ5-iodanuide (ref 16)
(b) hexafluoro-λ7-iodanide (ref 16) |
| F8Te2- | (a) octafluoro-λ6-tellanediuide (ref 16)
(b) octafluoro-λ10-tellanediide (ref 16) |
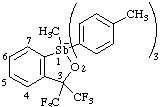
(a) 1,3-dihydro-1-methyl-1,1,1-tri-p-tolyl-3,3-bis(trifluoromethyl)-1λ5-benzo[c][1,2]-oxastibol-1-uide (ref 16)
(a) 1,3-dihydro-1-methyl-1,1,1-tri-p-tolyl-3,3-bis(trifluoromethyl)-2,1λ5-benzooxastibol-1-uide (ref 3h, 16)
Note: For recommendations to be published (ref 5) on elision in names of fused ring systems, see the note to example 4 under RC-81.3.3.2.
RC-83.3. Replacement nomenclature for anions. Anionic centers in parent hydrides may be described by the principles of replacement ("a") nomenclature (ref 2kk, 3g, 3i, 4q). Anionic replacement prefixes are derived by replacing the final "e" of the name of the mononuclear parent hydride (see RC-80.9.1) by the operational suffixes "-ida" or "-uida", and indicate an anionic center having a bonding number one lower (see RC-83.1) or one higher (see RC-83.2), respectively, than the bonding number of the corresponding neutral mononuclear parent hydride.
Note 1: In the 1979 IUPAC Organic Rules, replacement prefixes for describing anionic skeletal atoms with a bonding number one higher than that of the corresponding neutral heteroatom had the ending "-ata" (ref 4s, 4t).
Note 2: The 1979 IUPAC Organic Rules (ref 1) make no provision for replacement prefixes to describe anionic skeletal atoms with bonding numbers one lower than that of the corresponding neutral heteroatom.
Examples:
Anionic replacement prefixes are nondetachable, i.e., they are cited directly in front of the name of the parent hydride (ref 2f).
Anionic replacement prefixes are cited and the skeletal atoms they represent and are numbered, where there is a choice, in the same order as the corresponding neutral replacement ("a") prefixes given by Table I in Rule B-1.1 (ref 3d) or in Table I in the Appendix to Section D of the 1979 IUPAC Organic Rules (ref 4d). An anionic replacement prefix is cited immediately after the corresponding neutral replacement prefix (ref 3i) occurring in the same name; where there is a choice, an anionic skeletal atom is preferred to the corresponding neutral skeletal atom for assignment of low locants. An "-uida" anionic prefix is cited after the corresponding "-ida" prefix; where there is a choice, an "-uida" anionic center is preferred to an "-ida" anionic center for assignment of low locants. 
Note: In general, the Commission favors the use of the suffixes "-ide" and "-uide" with nonreplacement names of parent hydrides or with neutral replacement names according to RC-83.2 over the use of "ida" or "uida" replacement prefixes. In addition, names that do not require designation of skeletal atoms in nonstandard valency states by the l-convention (ref 16) are preferred (see also Note 5 to RC-82.2.2.2 and the Note to RC-82.4).
Examples:
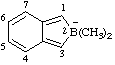 | 2,2-dimethyl-2H-2-boranuidaindene
2,2-dimethyl-2H-2-borainden-2-uide [RC-83.2(a)] |
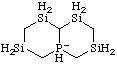 | decahydro-4a-phosphanuida-1,3,6,8-tetrasilanaphthalene
decahydro-4aλ5-phosphanida-1,3,6,8-tetrasilanaphthalene
1-phosphanuida-3,5,7,9-tetrasilabicyclo[4.4.0]decane
1λ5-phosphanida-3,5,7,9-tetrasilabicyclo[4.4.0]decane
octahydro-2H-4a-phospha-1,3,6,8-tetrasilanaphthalen-4a-uide [RC-83.2(a)]
1-phospha-3,5,7,9-tetrasilabicyclo[4.4.0]decan-1-uide [RC-83.2(a)]
decahydro-4aλ5-phospha-1,3,6,8-tetrasilanaphthalen-4a-ide [RC-83.2(b)]
1λ5-phospha-3,5,7,9-tetrasilabicyclo[4.4.0]decan-1-ide [RC-83.2(b)] |
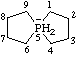 | 5λ5-phosphanuidaspiro[4.4]nonane
5λ5-phosphaspiro[4.4]nonan-5-uide [RC-83.2(a)] |
References for this section
1. International Union of Pure and Applied Chemistry. Organic Chemistry Division. Commission on Nomenclature of Organic Chemistry, Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F and H, 1979 ed., Pergamon Press, Oxford, 1979, 559 p.
2. Reference 1, Section C, pp. 79-322: [f] Rule C-16.1, p. 108; [kk] Subsection C-0.6, pp. 123-7.
3. Reference 1, Section B, pp. 53-76: [d] Rule B-1.1, pp. 53-5, Table I, p. 53; [g] Rule B-4, p. 68-70; [h] Rule B-3.5, pp. 67-8; [i] Rules B-6. pp. 71-2; B-10.1 (in part), p. 72; and B-11 (in part).
4. Reference 1, Section D, pp. 323-471: [d] Appendix, Table I, pp. 459-60; [g] Rule D-5.11, p. 384, footnote; [p] Rule D-5.71, p. 406; [q] Rule D-1.6, pp. 334-15; [s] Rule D-7.63, pp. 449-50; [t] Rule D-1.63, p. 336.
5. International Union of Pure and Applied Chemistry. Organic Chemistry Division. Commission on Nomenclature of Organic Chemistry, A Guide to IUPAC Nomenclature of Organic Compounds, Recommendations 1993, Blackwell Scientific Publications, Oxford, 1993.
6. International Union of Pure and Applied Chemistry. Inorganic Chemistry Division. Commission on Nomenclature of Inorganic Chemistry, Nomenclature of Inorganic Chemistry, Recommendations 1990, G. J. Leigh, ed., Blackwell Scientific Publications, Oxford, England, 1990, 289 p. [a] Sections I-8.3.3.5, p. 111; and I-11.5.1, pp. 232-3; [b] Section I-7.2.2.1, pp. 83-5, Table 17.2; [j] Chapter I-10, pp. 143-206; [k] Section I-8.3.3.6, p. 111.
16. International Union of Pure and Applied Chemistry. Organic Chemistry Division. Commission on Nomenclature of Organic Chemistry, Treatment of Variable Valence in Organic Nomenclature (Lambda Convention), Recommendations 1983, Pure Appl. Chem. 56, 769-78 (1984).
Continue with RC-83.4. Multiple anionic centers
Return to ions and radicals home page.
Return to chemical nomenclature home page.