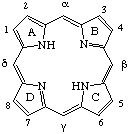
Fig. 1. Fischer numeration.
Continued from tetrapyrrole nomenclature homepage.
Preface
Introduction
References for this section
In 1968 the IUPAC Commission on the Nomenclature of Organic Chemistry (CNOC) and the IUPAC-IUB Commission on Biochemical Nomenclature (CBN) set up a Joint Subcommission to examine, and to make recommendations on, the nomenclature of tetrapyrroles (excluding hemoproteins). This group consisted of Professor S. Aronoff, Professor R. Bonnett, Dr. L. C. Cross, Dr. K. L. Loening and Professor C. Rimington, with Professor M. R. Lemberg as Convenor. The convenorship passed to Professor R. Bonnett in 1971, and two new members, Professors A. Eschenmoser and H. H. Inhoffen, joined the group.
The IUPAC-IUB Commission on Biochemical Nomenclature was replaced by the IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN) while the provisional recommendations were being prepared. During this process the Subcommission received and considered the views of many chemists and biochemists. Informal discussion sessions were held at a meeting on porphyrin chemistry in New York in 1972 [ref 1] and at a meeting on bile pigment biochemistry at Remsedal, Norway [ref 2]. We appreciate the cooperation of the organisers of these meetings in making arrangements for these sessions.
The provisional recommendations on the nomenclature of tetrapyrroles were published in 1979 [IUPAC-IUB Joint Commission on Biochemical Nomenclature, "Nomenclature of Tetrapyrroles, recommendations 1978", Pure Appl. Chem. 51, 2251-2304 (1979); Eur. J. Biochem. 108, 1-30 (1980)]. They have since been widely adopted and the present revision is based on comments received on the provisional document. In the revised recommendations two new trivial names (isobacteriochlorin and sirohydrochlorin) are defined, the names of linear tetrapyrroles are amended, and the system is extended to dipyrrole systems. Tables are provided to show the structures of the more commonly encountered compounds using the Fischer system for denoting isomers (the fifteen isomers of mesoporphyrin defined by Fischer, and some isomers of biliverdin). Experience has shown that these names continue to be useful because of their brevity. The revision was formulated by Professor R. Bonnett in consultation with Professor S. Aronoff, Professor A. R. Battersby, Professor A. Eschenmoser, Professor H. Falk, Professor A. Gossauer, Professor H. H. Inhoffen, Professor A. H. Jackson, Professor D. A. Lightner, Professor S. Onishi, Dr J. M. Ribo, Professor C. Rimington, Dr M. S. Stoll and a subcommittee of the IUPAC-IUB Joint Commission on Biochemical Nomenclature composed of Professor P. Karlson, Dr K. L. Loening and Dr G. P. Moss.
"Tetrapyrrole" is a term used widely, but loosely, to refer to a member of a class of compounds whose molecules have four rings of the pyrrole type, generally linked together by single-atom bridges between the alpha positions of the five-membered pyrrole rings. The common arrangements of the four rings for which this name is used are macrocyclic (as in the porphyrins) and linear (as in the bile pigments).
Note The alpha positions of the pyrrole ring are those adjacent to the nitrogen atom. This use of "alpha" should not be confused with its stereochemical meaning.
Tetrapyrroles and their relatives are of importance in several disciplines. The international nomenclature commissions have not previously put forward specific recommendations for this field. The nomenclature most generally used has been that of Fischer [ref 3,4] which is based on a very large number of trivial names which continue to appear (e.g. pemptoporphyrin [ref 5], harderoporphyrin [ref 6]) and a numeration scheme, shown in Fig. 1 for the unsubstituted porphyrin ring system, which is simple but incomplete. The availability of a more systematic nomenclature would be expected to help interdisciplinary communication, and would considerably diminish the need for new trivial names. See also Table 1.

Fig. 1. Fischer numeration.
These recommendations provide for naming porphyrins, hydroporphyrins, ring contracted or expanded porphyrins, porphyrins fused with other rings, skeletally replaced porphyrins and porphyrin-metal coordination complexes together with corresponding linear arrangements of three and four pyrrole rings. The application of these recommendations permits these substances to be named more systematically using fewer trivial names than are now currently used in the literature. Fundamental porphyrin and bilin nuclei form the basis of a systematic tetrapyrrole nomenclature in accord with the recommendations of the provisional rules for natural products. See IUPAC, "Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F and H, 1979 edition." Pergamon, 1979, Section F. [Revised 1999]
In parallel, a semisystematic tetrapyrrole nomenclature is developed based on a few trivial names, selected mainly from the Fischer system, that are so well established that is is desirable to retain them. The general rules for substitutive nomenclature, including operations of replacement, substitution and subtraction, are applied as appropriate. The application of these recommendations makes the use of a large number of trivial names no longer necessary. Trivial names are not recommendedc except for the select few retained as the basis of the systematic and semisystematic tetrapyrrole nomenclature systems. See Appendix 1.
Note Some of the Fischer trivial names (see Appendix 3) are likely to persist, for example, in the discussion section of a paper. This is regarded as permissible provided that the systematic or semisystematic name appears by way of definition at least once in each paper.
In addition, the incomplete Fischer numeration is abandoned for the porphyrin nucleus and the corresponding skeleton of the bile pigments. The 1-24 numbering scheme, already proven over an eighteen-year period with the closely related nucleus of the corrinoids, is adopted as shown in TP-1.1 Fig. 2. A comparison of the 1-24 numbering scheme with the Fischer numeration for the unsubstituted porphyrin nucleus is given in Table 1.
See IUPAC-IUB Commission on Biochemical Nomenclature, "Nomenclature of Corrinoids, Rules Approved 1975." Pure Appl. Chem. 48, 495-502 (1976) which supersedes IUPAC Commission on the Nomenclature of Biological Chemistry, "Definitive Rules for the Nomenclature of Vitamins." J. Am. Chem. Soc. 82, 5582 (1960), Rule V-15.
Table 1. Comparison of the 1-24 numbering scheme with the Fischer numeration for the unsubstituted porphyrin nucleus.
| Recommended: | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 |
| Fischer: | - | 1 | 2 | - | α | - | 3 | 4 | - | β | - | 5 | 6 | - | γ | - | 7 | 8 | - | δ | - | - | - | - |
Even though the 1-24 numbering scheme is used for the porphyrin and bilin nuclei, the numbering of the point of attachment of substituents on the nucleus may be different for systematic tetrapyrrole nomenclature than for semisystematic tetrapyrrole nomenclature. In systematic tetrapyrrole nomenclature the beginning and direction of numbering is determined by the rules for systematic substitutive nomenclature. Lowest locants are assigned according to the criteria of principal group named as suffix, substituents first cited, etc. In contrast, in semisystematic tetrapyrrole nomenclature, the beginning and direction of numbering are the same as those established by Fischer for the trivially named porphyrins. A comparison of the systematic and trivial numeration based on the 1-24 numbering scheme is presented in Appendix 2 for substituted porphyrins for which trivial names have been retained.
Note 1 IUPAC, Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F and H, 1979 edition. Pergamon, 1979, Rule C-15.1. See also TP-1.7.Note 2 See Appendix 1.
1. R. Bonnett, Ann. N. Y. Acad. Sci., 206, 745 (1973).
2. R. Bonnett, Proceeding of 9th Meeting of the European Association for the Study of Liver Diseases, Hemsedal (1974) p. 212.
3. H. Fischer-and H. Orth, Die Chemie des Pyrrols, Volume II.1, Akademische Verlagsgessellschaft, Leipzig (1937).
4. H. Fischer and A. Stern, Die Chemie des Pyrrols, Volume II.2, Akademische Verlagsgessellschaft, Leipzig, (1940).
5. S. Sano, T. Shingu, J. M. French and E. Thonger, Biochem. J., 97, 250 (1965).
6. G. Y. Kennedy, A. H. Jackson, G. W. Kenner and C. J. Suckling, FEBS Letters, 6, 9 (1970).