Contents of this section
Replacement of skeletal atoms in parent structures whose names are formed according to RF-3 and RF-4 above is indicated by extending the principles of organic replacement nomenclature [see rules B-4 (ref 2), C-0.6 (ref 2) and R-2.3.3.2 (ref 3)].
RF-5.1. Replacement of a Carbon Atom in a Parent Structure
The replacement of a carbon atom in the skeletal system of a parent structure by a heteroatom is described by appropriate replacement "a" prefixes [see R-9.3 (ref 3)]. This procedure is used even though the parent structure may already be a heterocycle. The numbering of the parent structure is retained.
Examples:
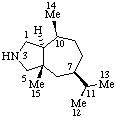 | 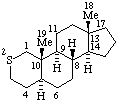 |
| 3-Azaambrosane | 2-Thia-5α-androstane |
6,8-Dioxamorphinan
RF-5.2. Replacement of a Heteroatom in a Parent Structure
The replacement of a heteroatom in a parent structure of a natural product by a carbon atom is described by the replacement prefix "carba". The original numbering is retained. If the heteroatom in the parent structure is unnumbered, the replacing carbon atom is numbered by affixing the letter "a" to the locant of the immediately adjacent lower numbered skeletal atom. If the immediately adjacent lower numbered skeletal atom is a "homo" atom, the letter "b", "c", etc., as appropriate is used. Stereochemical configuration at the new carbon skeletal atom is described by methods for specifying additional stereochemistry (see RF-10.2).
Examples:
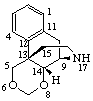
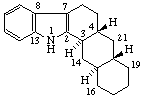 | 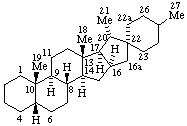 |
| (4βH)-4-Carbayohimban | 16a,22a-Dicarba-5β-spirostan |
RF-5.3. Replacement of a Heteroatom in a Parent Structure by Another Heteroatom
Replacement of a heteroatom in a parent structure by another heteroatom is denoted by the appropriate replacement ("a") prefix.
Example:
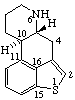
When the replacement of a skeletal atom in a portion of the structure of a fundamental parent structure that contains the maximum number of noncumulative double bonds or an extended conjugated system of double bonds results in the creation of a saturated skeletal position, that position is indicated by the symbolism of indicated hydrogen.
Examples:
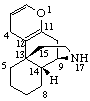 | 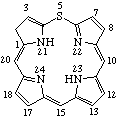 |
| 4H-1-Oxamorphinan | 2H-5-Thiaporphyrin (the name porphyrin implies two saturated positions normally shown at 21 and 23) |
2. International Union of Pure and Applied Chemistry, Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F, and H, l979 edition, Pergamon Press, Oxford, 1979.
3. International Union of Pure and Applied Chemistry, A Guide to IUPAC Nomenclature of Organic Compounds, Blackwell Scientific Publications, Oxford, 1993. [Corrections see Pure Appl. Chem., 71, 1327-1330 (1999).]