Contents of this section
It is often convenient to retain the advantages of a semisystematic natural product name, particularly with regard to stereochemistry, for naming structures having rings or ring systems fused to a fundamental parent structure of a natural product.
Since most natural products with cyclic structures have fundamental parent structures that are fully saturated and fusion nomenclature principles are based on the concept of the presence of the maximum number of noncumulative double bonds, adaptations of general fusion nomenclature are necessary. These adaptations are described in the recommendations that follow. No attempt is made to legislate rigidly or to cover every case. The decision between a fusion-natural product name and a systematic fusion name is left to choice based on particular circumstances in each case.
RF-6.1. Rings Derived from Functional Groups
Rings or ring systems fused to a fundamental parent structure of a natural product when derived from functional groups, such as lactones or cyclic acetals, are preferably named by the usual methods of organic nomenclature for such cyclic functional groups (see also RF-6.2.2 and RF-9.2).
Examples:
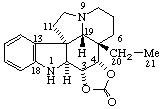 | 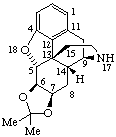 |
| Aspidospermidine-3α,4α-diyl carbonate | Acetone 4,5α-epoxymorphinan-6β,7β-diyl ketal (for use of epoxy as a bridge, see RF-7) |
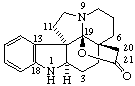
21-Noraspidospermidine-20,19-carbolactone
(also named 19-Hydroxyaspidospermidineo-21,19-lactone
RF-6.2. Fundamental Parent Structure Saturation or Unsaturation
The fundamental parent structure of the natural product as a component is used in its normal state of saturation or unsaturation. Accordingly, a double bond is not cited in the natural product parent at the fusion site just because the other component contains the maximum number of noncumulative double bonds. Hence, the bonding at a fusion site is governed usually by the nonnatural product component; fusion resulting in a quaternary carbon atom at the fusion site also affects the bonding (see RF-6.2.3, below).
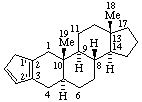
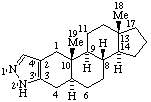
RF-6.2.1. A ring or ring system of systematic organic nomenclature (ref 2) (carbocyclic or heterocyclic) fused to a fundamental parent structure is described by its fusion prefix name (see A-21.4 and B-3) (ref 2), R-2.4.1.1 (ref 3), FR-2.1 and FR-2.2 (ref 7) prefixed to the name of the fundamental parent structure. The position of the fusion is indicated by sets of locants, as needed, separated by a colon, enclosed in square brackets, and inserted into the name between the components. The skeletal atoms of the natural product are identified by plain (unprimed) locant numbers and the "systematic" component by primed locant numbers. Where there is a choice, the locant numbers for the systematic component are as low as possible and are cited in the same direction of numbering as for the natural product component. Terminal vowels of the names of systematic components are not elided when followed by a vowel [this is consistent with the recommendation contained in the fused ring nomenclature report (ref 7) and is a change from previous recommendations (ref 2)].
Examples:
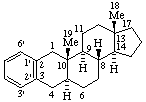 | 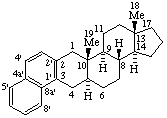 |
| Benzo[2,3]-5α-androstane (note that only one set of locants, that of the anrostane component, is needed here; the CA Index name is Benz[2,3]androst-2-ene, (5α)-) | Naphtho[2',1':2,3]-5α-androstane |
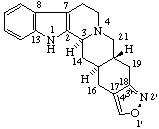 | 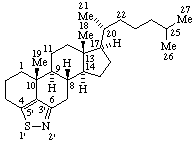 |
| 1H-[1,2]Oxazolo[4',3':17,18]yohimban | [1,2]Thiazolo[5',4',3':4,5,6]cholestane |
When two or more identical systematic components are fused to a natural product component primes are assigned to the systematic component attached to the lower numbered natural product positions, double primes to the next component, etc. When there are two or more nonidentical systematic components these are cited in alphabetical order and primes are assigned in the same order.
Examples:
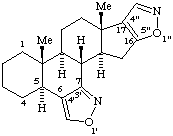
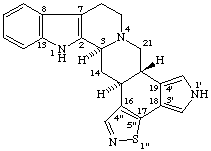
1'H-Pyrrolo[3',4':18,19][1,2]thiazolo[4",5":16,17]yohimban
RF-6.2.2. The systematic organic component fused to the fundamental parent component of a natural product structure contains the maximum number of noncumulative double bonds consistent with the bonding requirements at the fusion site. If it is necessary to identify saturated positions on the systematic component (including the fusion sites) that have at least one hydrogen present, these are designated by the indicated hydrogen method [see A-21.6 (ref 2) and R-1.3 (ref 3)]. Unprimed locants are used to identify the position of the indicated hydrogen, where there is a choice. For configuration at chirality centres which arise from the fusion process see RF-6.2.4.
Examples:
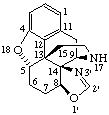
 |  |
| 5'H-Cyclopenta[2,3]-5α-androstane | 2'H-Pyrazolo[4',3':2,3]-5α-androstane |
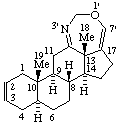
2'H-[1,3]Oxazepino[4',5',6':12,13,17]-5α-androstane
RF-6.2.3. Saturated, or partially saturated, carbocyclic and heterocyclic ring components fused to the fundamental parent structure of a natural product are named using hydro prefixes. Where there is a choice, the unprimed locants are used.
Examples:
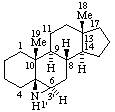 | 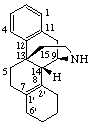 |
| (6αH)-1',6-Dihydroazirino[2',3':5,6]-5β-androstane | 3',4',5',6'-Tetrahydrobenzo[7,8]morphinan |
RF-6.2.4. The configuration at chirality centres which arise from the fusion process or their hydrogenated derivatives is indicated by the α/β system in accordance with its use in steroid nomenclature [see 3S-1.4, 3S-10.2 (ref 4) and RF-10], or, if necessary by the Sequence Rule method (R/S) (see also RF-10). The projection of the hydrogen atom below (α) or above (β) the reference plane of the ring system is indicated by the locant and the appropriate symbol, and a capital italic letter H, all cited within parentheses. Note that such a stereodescriptor does not replace indicated hydrogen. (See also examples in RF-6.2.2 and RF-6.2.3).
Examples:
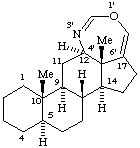
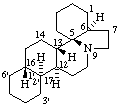
(12αH,13βH,16βH,17αH)-3',4',5',6',12,13,14,15,16,17-Decahydrobenzo[16,17]erythrinan
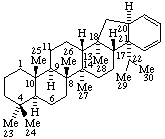
(20αH,21R)-20H-benzo[20,21]17(2221)-abeogammacerane
(also has been named as a 1'H-Benzo[20,21]-A'-neogammacerane)
2. International Union of Pure and Applied Chemistry, Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F, and H, l979 edition, Pergamon Press, Oxford, 1979.
3. International Union of Pure and Applied Chemistry, A Guide to IUPAC Nomenclature of Organic Compounds, Blackwell Scientific Publications, Oxford, 1993. Corrections Pure Appl. Chem., 71, 1327-1330 (1999).
4. International Union of Pure and Applied Chemistry and International Union of Biochemistry, Joint Commission on Biochemical Nomenclature, "Nomenclature of Steroids", Pure Appl. Chem. 61, 1783-1822 (1989). [also in: Eur. J. Biochem. 186, 429-458 (1989) and pages xxx-lix in Dictionary of Steroids (Hill, R.A., Kirk, D.N., Makin, H.L.J. & Murphy, G.M., eds) Chapman & Hall, London 1991].
7. International Union of Pure and Applied Chemistry, "Nomenclature of Fused and Bridged Fused Ring Systems", Pure Appl. Chem., 70, 143-216 (1998).