The modifications to a fundamental parent structure prescribed by the prefixes in the preceding recommendations (RF-4.1 through RF-4.4, i.e. excluding bridges, fusion and detachable prefixes) may be combined to effect even more drastic changes in structure. The operation indicated by each prefix is applied to the fundamental parent structure sequentially as one "advances backwards", i.e., moves from right to left, from the name of the fundamental parent structure. The following recommendations are not rigorous rules for choosing a unique name, but are intended to be guidelines for choosing combinations of prefixes and for the order of citation in generating an unambiguous name.
RF-4.7.1. When different combinations of prefixes can be used to effect the same transformation in a parent structure, the combination of choice should express the fewest number of operations. Both detachable (e.g. alkyl) and non-detachable (e.g. homo or nor) prefixes are considered as modifications but detachable prefixes are preferred. Dihomo, dinor, etc., are counted as two modifications each [see Rule 3S-6.3 (ref 4)]. When the number of operations is the same, the combination of homo/nor is preferred to cyclo/seco; choice between other combinations expressing the same number of operations is based on earliest alphabetic order of the prefixes.
Example:
Example:
Examples:
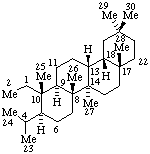
 | 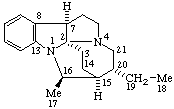 |
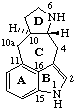
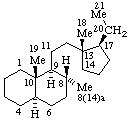
| 13(17)a-Homo-12,18-dinor-5α-pregnane | (2S,7αH,16αH)-7H-1,16:2,4-Dicyclo-3,4-secocorynan |
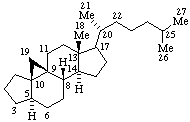 |  |
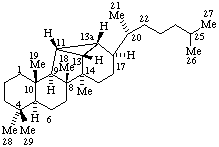
| 9,19-Cyclo-4-nor-5α,9β-cholestane | 11β,18-Cyclo-12a,12b-dihomo-5α-pregnane |
Note: The cyclodihomopregnane example above can be named in at least two other ways: (1) 11α,18β-Cyclo-18a,18b-dihomo-5α,13α-pregnane; (2) 11α,13-Propano-18-nor-5α,13α-pregnane. The first name uses the same number of operations, but extends a side chain rather than enlarging a ring (the latter operation seems more usual) resulting in higher locant numbers. The second name uses only one operation of the type discussed in this section, but requires the use of a bridge (see RF-6), which may or may not be considered preferable.
| bond rearrangement | removal/addition of skeletal atoms | Parent structure |
| cyclo, seco | apo, homo, nor |
Examples:
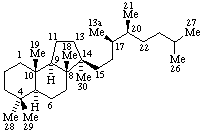
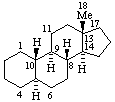 | 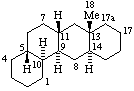 |
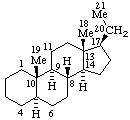
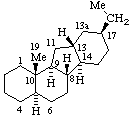
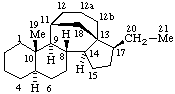
| Estrane (fundamental parent structure) | 7,11α-Cyclo-7,8-seco-17a-homo-5α-estane (7,11α-Cyclo-17a-homo-7,8-seco-5α-estrane by RF-4.7.3) |
Note. This example can also be named (11βH)-7(811)-Abeo-17a-homo-5α-estrane which uses only two operations.
4. International Union of Pure and Applied Chemistry and International Union of Biochemistry, Joint Commission on Biochemical Nomenclature, "Nomenclature of Steroids, Recommendations 1989", Pure Appl. Chem. 61, 1783-1822 (1989). [also in: Eur. J. Biochem. 186, 429-458 (1989) and pages xxx-lix in Dictionary of Steroids (Hill, R.A., Kirk, D.N., Makin, H.L.J. & Murphy, G.M., eds) Chapman & Hall, London 1991].