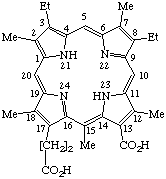
Systematic: 18-Carboxy-8,13-diethyl-3,7,12,17,20-
-pentamethylporphyrin-2-propionic acid
Fischer: Chloroporphyrin e4
Continued from TP-2 Trivial Names
TP-3 Semisystematic porphyrin names
TP-3.1 Semisystematic names in substituted porphyrins
TP-3.2 Subtractive nomenclature
TP-3.3 Combinations of substitutive and subtractive operations
TP-3.4 Additional ring formation
TP-3.5 Skeletal replacement of substituted porphyrins
TP-3.1. Semisystematic Names For Substituted Porphyrins. As an alternative to a systematic name based on the porphyrin ring nucleus, compounds closely related to the trivially named porphyrins listed in Rule TP-2, Table 2 and Table 3, but differing in that one or more hydrogen atoms have been replaced (substituted) by one or more other atoms or groups, may be named semisystematically based on one of the trivially named parent porphyrins. Prefixes, and/or a suffix of the new substituents are combined with the trivial name of the parent porphyrin.
Note 1. See TP-1.7 for generation of systematic names.Note 2. Precise guidelines for naming modified or substituted side chains of trivially named natural products are needed. In general, chain lengthening of substituent groups by substitution of hydrogen atoms by alkyl groups is less preferred than removal of the entire side group and the addition of the new group. See TP-3.2 for subtractive nomenclature.
Note 3. See IUPAC Rule C-10.3.
The functional group of highest priority, not implied in the trivial parent name, may be expressed as a suffix. The remaining substitutents not implied by the trivial name are expressed by prefixes. Locants and multiplying prefixes are added as appropiate, and the prefixes arranged in alphabetical order.
When there is a choice among trivially named parent porphyrins, the one with the highest possible rank number in Table 2 is preferred. However a porphyrin of lower rank may be selected where special emphasis is desired.
Functional derivatives such as esters and lactones are named similarly and in accord with the rules for organic nomenclature.
Note See Rule C-4.6 and C-4.7. See also TP-3.4.
Examples:
| 1. |  | Semisystematic: 15-Methylrhodoporphyrin Systematic: 18-Carboxy-8,13-diethyl-3,7,12,17,20- Fischer: Chloroporphyrin e4 |
Note 1. Not phylloporphyrin-13-carboxylic acid. Rhodoporphyrin has a higher rank number than phylloporphyrin.Note 2. See TP-1.7 for generation of systematic names.
| 2. | 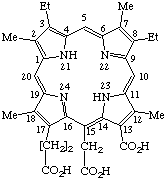 | Semisystematic: Rhodoporphyrin-15-acetic acid Systematic: 18-Carboxy-20-(carboxymethyl)-8,13-diethyl- Fischer: Chloroporphyrin e6 |
Note 1. Note that in contrast to regular practices of substitutive nomenclature that the propionic principal group is unexpressed. In these rules a subordinate group may be cited as a suffix. Compare IUPAC Rule C-10.3.
| 3. | 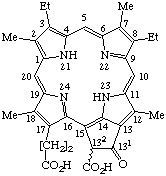 | Semisystematic: Phytoporphyrin-132-carboxylic acid Systematic: 22-Carboxy-7,12-diethyl-21,22-dihydro- Fischer: Pheoporphyrin a5 |
Note if it is necessary to specify the absolute stereochemistry of this molecule, it should be indicated by R or S in accord with TP-0.3.
| 4. | 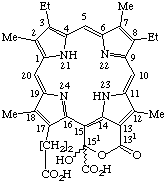 | Semisystematic: 151,151-Dihydroxyrhodoporphyrin-15-acetic acid δ-lactone Systematic:18-Carboxy-20-(carboxydihydroxymethyl)-8,13-diethyl- Fischer: Pheoporphyrin a7 |
Note 1. Note that in contrast to regular practices of substitutive nomenclature that the propionic principal group is unexpressed. In these rules a subordinate group may be cited as a suffix. Compare IUPAC Rule C-10.3.Note 2. If it is necessary to specify the absolute stereochemistry of this molecule, it should be indicated by R or S in accord with TP-0.3.
Note 3. For systematic numbering see TP-1.1 and TP-1.7.
Note 4. Alternatively a name based on ring fusion principles could be formed, but see footnote d, TP-1.3.
| 5. | 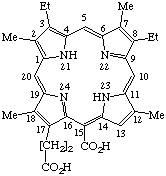 | Semisystematic: Phylloporphyrin-151-oic acid Systematic: 20-Carboxy-8,13-diethyl-3,7,12,17- Fischer: γ-Carboxypyrroporphyrin |
Note Not Pyrroporphyrin-15-carboxylic acid. Phylloporphyrin ranks higher than pyrroporphyrin in Table 2.
| 6. | 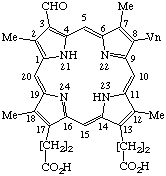 | Semisystematic: 3-Formy1-8-vinyldeuteroporphyrin Systematic: 8-Formyl-3,7,12,17-tetramethyl-13- Fischer: Chlorocruoroporphyrin or |
| 7. | 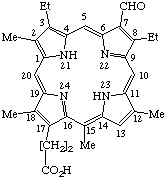 | Semisystematic: 71-oxophylloporphyrin Systematic: 8,13-Diethyl-12-formyl-3,7,17,20- Fischer: Rhodinporphyrin g3 |
Note 1. Further development of guidelines for choice of the trivially named parent porphyrin, its numbering, and limits of application of these operations is needed.Note 2. See IUPAC Rule C-41.
Note 3. See IUPAC provisional Rules F-4.10 and F-4.13.
Note 4. See IUPAC Rule C-15.11(d) and (e).
Example:
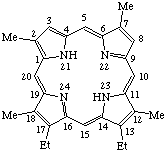 | Semisystematic: 3,8-Dideethyletioporphyrin III Systematic: 3,7-Diethyl-2,8,12,17-tetramethylporphyrin Fischer: Deuteroetioporphyrin IX |
Note See TP-1.7 for formation of the systematic name.
TP-3.3. Combinations of Substitutive and Subtractive Operations. The subtractive operations of TP-3.2 may be combined with the operations of substitution of TP-3.1 to denote the removal of a group or atom from a position in the parent porphyrin and subsequent substitution at any position. The subtractive prefixes are alphabetized among the substitutive prefixes. As low locants as possible are selected for substitutive and subtractive prefixes considered together in one series. If this is not decisive, then the numbering is chosen that assigns the lowest locant to the first cited substituent or subtractive prefix. When a porphyrin structure can be considered as a derivative of two or more trivially named parent porphyrins listed in Tables 2 and Tables 3, and shown in Appendix 1, the name that requires the fewest number of subtractive and substitutive operations combined is preferred. If this is not decisive, then the name that requires the smaller number of subtractive prefixes is preferred.
Example:
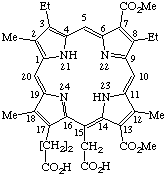 | Semisystematic: 7-Demethyl-7-(methoxycarbonyl)rhodoporphyrin- -15-acetic acid 13-methyl ester Systematic: 20-(Carboxymethyl)-8,13-diethyl-12,18,bis(methoxycarbonyl)- Fischer: Rhodinporphyrin 88 |
Note Alternate formats for naming this diester should be considered such as 7-carboxy-7-demethylrhodoporphyrin-15-acetic acid 7,13-dimethyl ester, or 13-methyl 7-demethyl-7-(methoxycarbonyl)rhodoporphyrin-15-acetate. Present IUPAC nomenclature rules are not definitive in this respect.
TP-3.4. Additional ring formation. Compounds closely related to the trivially named porphyrins of Rule TP-2 but in which an additional ring has been formed by means of a direct link between two atoms of the parent structure are named by prefixing "cyclo" preceded by the locants of the positions joined by the new bond. Alternatively such compounds may be named according to the provisions for fused rings in Rule TP-1.3.
Note See IUPAC provisional Rule F-4.1.
Examples
Note 1. If it is necessary to specify the absolute stereochemistry of this molecule, it should be by R or S according to TP-0.3.Note 2. See TP-1.3 for the systematic name and numbering of the fundamental ring.
TP-3.5. Skeletal Replacement of Substituted Porphyrins. Trivially named porphyrins in which one or more of the atoms in the porphyrin ring nucleus have been replaced by atoms of other elements are named by prefixing to the trivial name, the appropriate locants with corresponding multiplicative and replacement prefixes. Where there is a choice due to the symmetry of substituents, the replacing atoms are numbered in line with IUPAC Rule B-4.
Example:
Note See. TP-1.4 and TP-1.5 for systematic names and numbering of skeletally replaced fundamental porphyrin systems.
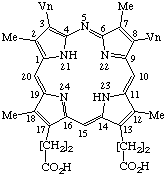 | Semisystematic: 5-Azaprotoporphyrin Systematic: 2,7,12,18-Tetramethyl-3,8-divinyl- |