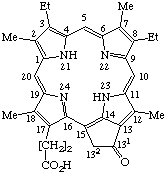
Fig. 5. Phytoporphyrin.
Continued from TP-0 and TP-1 General and Fundamental Porphyrin Systems
TP-2 Trivial names and locants for certain substituted porphyrins
TP-2.1 Trivial names and locants
TP-2.2 Roman numeral type notation
Note See also TP-4.3 and TP-4.4 for trivial names of substituted reduced porphyrins including pheophorbides, pheophytins and chlorins.
TP-2.1. Trivial Names and Locants. Eleven well-established trivial names and associated locants are retained for naming certain substituted porphyrin structures containing the porphyrin nucleus. They are listed in Tables 2 and Tables 3. They are also explicitly shown in Appendix 1. In addition to these names the name phytoporphyrin is recommended for the hexacyclic structure numbered as shown in Fig. 5. Phylloerythrin, the name formerly used for this structure, is abandoned.
Note 1. The locants used with these trivial names are retained for semisystematic porphyrin names formed according to TP-3.1. However, they do not coincide with those used for systematic porphyrin names formed according to TP-1.7. A comparison of the locants of trivially named porphyrins with locants derived systematically is given in Appendix 2.Note 2. With the exception of the etioporphyrins, the structures denoted by these trivial names do not meet the criteria of IUPAC Rule F-2 regarding selection of parent structures for semisystematic names. However because of their firm establishment in the porphyrin field, these eleven names are carried over from the Fischer system. These names form the basis of a semisystematic nomenclature for naming other porphyrin derivatives.

Fig. 5. Phytoporphyrin.
Note A systematic porphyrin name of phytoporphyrin based on the fundamental ring named as in TP-1.3 would be: 7,12-Diethyl-21,22-dihydro-3,8,13,17-tetramethyl-21-oxocyclopenta[at]porphyrin-18-propionic acid.
Table 2. Trivial Names for Substituted Porphyrins a
| Trivial Nameb | Rank | Substituentsc and locants | ||||||||
| 2 | 3 | 7 | 8 | 12 | 13 | 15 | 17 | 18 | ||
| Coproporphyrin I | 9 | Me | Cet | Me | Cet | Me | Cet | H | Me | Cet |
| Cytoporphyrin | 11 | Me | -CH(OH)CH2R' | Me | Vn | Me | Cet | H | Cet | -CHO |
| Deuteroporphyrind,e | 1 | Me | H | Me | H | Me | Cet | H | Cet | Me |
| Etioporphyrin I | 3 | Me | Et | Me | Et | Me | Et | H | Me | Et |
| Hematoporphyrine | 8 | Me | -CH(OH)CH3 | Me | -CH(OH)CH3 | Me | Cet | H | Cet | Me |
| Mesoporphyrine | 7 | Me | Et | Me | Et | Me | Cet | H | Cet | Me |
| Phylloporphyrinf | 4 | Me | Et | Me | Et | Me | H | Me | Cet | Me |
| Protoporphyrine | 6 | Me | Vn | Me | Vn | Me | Cet | H | Cet | Me |
| Pyrroporphyrinf | 2 | Me | Et | Me | Et | Me | H | H | Cet | Me |
| Rhodoporphyrinf | 5 | Me | Et | Me | Et | Me | -CO2H | H | Cet | Me |
| Uroporphyrin I | 10 | Cm | Cet | Cm | Cet | Cm | Cet | H | Cm | Cet |
| Phytoporphyrin | 12 | Me | Et | Me | Et | Me | -C(O)-CH2- | Cet | Me | |
a The porphyrins are arranged alphabetically. They are ranked according to (1) number of component rings (2) number of carbon atoms, (3) molecular weight. A porphyrin of higher rank number is preferred to one of lower rank for selection as parent for semisystematic names according to TP-3.1.b For explanation of Roman numerals associated with some of the trivial names see TP-2.2, and Table 3.
c The following abbreviations are used; Cm for -CH2CO2H; Cet for -CH2CH2CO2H; Me for CH3; Et for -CH2CH3; Vn for -CH=CH2; see Eur. J. Biochem. 74, 1-6 (1977). The use of the symbols A and P, which is a parochial practice in the field of porphyrin chemistry for CH2CO2H and -CH2CH2CO2H, respectively, is not recommended.
trans,trans-farnesyl which is (2E,6E)-3,7,11-trimethyl-2,6,10-dodecatrienyl. See J. Chem.Soc. Chem. Commun. 1977, 278.d Not to be confused with deuterioporphyrin, a possible name for an isotopically labeled compound.
e Formerly type IX (see Appendix 3)
f Formerly type XV.
The side chains of trivially named porphyrins may be numbered using the number of the point of substitution in the porphyrin nucleus, with a following superscript numeral as shown in Fig. 6. The trivial names listed in Table 2 and TP-2.2, Table 3, may be used in deriving semisystematic names for other substituted porphyrins as directed in TP-3.1 and TP-3.2.
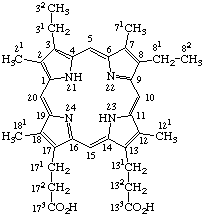
Fig. 6. Numbering of Mesoporphyrin.
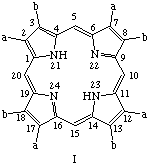
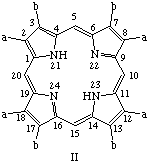
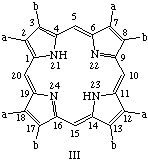
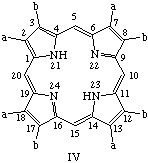
TP-2.2. Roman Numeral Type Notation. There are only four possible positional isomers for each of the 2,3,7,8,12,13,17,18-octasubstituted coproporphyrin, etioporphyrin and uroporphyrin structures in which (i) the substituents are of two sorts only, and (ii) one of each sort of substituent is present as a substituent in each of the component pyrrolic rings A, B, C and D. The four positional isomers are distinguished by Roman numerals, I, II, III or IV applied to these substituted porphyrin rings, numbered and oriented as shown below where substituent a is smaller than substituent b. The substituents and locants for these porphyrins are summarized in Table 3. The use of Roman numerals or "type nomenclature" is not recommended for more complex cases, or for porphyrins with more than four possible positional isomers.
Note 1. S. Aronoff, "The number of biologically possible porphyrin isomers," Ann. N.Y. Acad. Sci. 244, 327-333 (1975).Note 2. In orienting these trivially named structures the following guidlines were followed: (a) in ring A the sequence "smaller substituent-larger substituent" is clockwise; and (b) insofar as it is possible this order "smaller substituent-larger substituent" continues in turn for ring B, C, D, in that priority.
 |  |
 |  |
| Name | Substituents and locants | ||||||||
| 2 | 3 | 7 | 8 | 12 | 13 | 15 | 17 | 18 | |
| Coproporphyrin I | Me | Cet | Me | Cet | Me | Cet | H | Me | Cet |
| Coproporphyrin II | Me | Cet | Cet | Me | Me | Cet | H | Cet | Me |
| Coproporphyrin III | Me | Cet | Me | Cet | Me | Cet | H | Cet | Me |
| Coproporphyrin IV | Me | Cet | Me | Cet | Cet | Me | H | Cet | Me |
| Etioporphyrin I | Me | Et | Me | Et | Me | Et | H | Me | Et |
| Etioporphyrin II | Me | Et | Et | Me | Me | Et | H | Et | Me |
| Etioporphyrin III | Me | Et | Me | Et | Me | Et | H | Et | Me |
| Etioporphyrin IV | Me | Et | Me | Et | Et | Me | H | Et | Me |
| Uroporphyrin I | Cm | Cet | Cm | Cet | Cm | Cet | H | Cm | Cet |
| Uroporphyrin II | Cm | Cet | Cet | Cm | Cm | Cet | H | Cet | Cm |
| Uroporphyrin III | Cm | Cet | Cm | Cet | Cm | Cet | H | Cet | Cm |
| Uroporphyrin IV | Cm | Cet | Cm | Cet | Cet | Cm | H | Cet | Cm |
Note Abbreviations used are Me for "methyl;" Cet for "carboxyethyl;" Et for "ethyl;" Cm for "carboxymethyl;" see Eur. J. Biochem. 74, 1-6 (1977).