alumane (preselected name, see P-12.2)
(not alane)
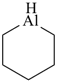
aluminane
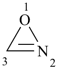
InH3
indigane (preselected name, see P-12.2)
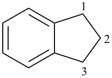
indane
2,3-dihydro-1H-indene (PIN)
Division VIII Chemical Nomenclature and Structure Representation Division
P-20 IntroductionP-20 INTRODUCTION
P-21 Mononuclear and acyclic polynuclear parent hydrides
P-22 Monocyclic parent hydrides
P-23 Polyalicyclic parent hydrides (extended von Baeyer system)
P-24 Spiro ring systems
P-25 Fused and bridged fused ring systems
P-26 Phane nomenclature
P-27 Fullerenes
P-28 Ring assemblies
P-29 Prefixes denoting substituent groups derived from parent hydrides
A parent hydride is the structure that is named before the addition of affixes denoting substituents to yield the name of a specific compound. Its name is understood to signify a definite population of hydrogen atoms attached to a skeletal structure. Acyclic parent hydrides are always saturated and unbranched, for example, pentane and trisilane. Cyclic parent hydrides are usually either fully saturated, for example, cyclopentane, cyclotrisiloxane, azepane, bicyclo[3.2.1]octane, and spiro[4.5]decane, or fully unsaturated, i.e., they contain the maximum number of noncumulative double bonds, for example benzene, pyridine, 1,3-oxazole, 1H-phenalene, phenanthroline, and benzo[a]acridine. Also, there are parent hydrides that are partially saturated, for example 1H-spiro[[1,3]-dioxolane-2,1′-indene], and there are combinations of cyclic and acyclic hydrides, having traditional retained names, for example toluene.
Names of parent hydrides that do not contain skeletal carbon atoms, for example trisilane, are not designated as preferred IUPAC names in these recommendations. Instead they are called preselected names, i.e., they are used to generate preferred IUPAC names for derivatives substituted by organic (carbon-containing) substituents (see P-12.2). They may, however, become IUPAC preferred names depending on decisions of a team formed to decide IUPAC preferred names for inorganic (noncarbon-containing) compounds.
Names of parent hydrides are either traditional names that are retained or systematic names formed in accordance with specific rules. Rules and names must be unambiguous and clear. In order to achieve this goal and keep rules simple and concise, the rules for selecting preferred IUPAC names and preselected names of parent hydrides and prefixes denoting substituent groups derived from parent hydrides are not provided in this Chapter. These rules are given in Chapter P-5.
P-21 MONONUCLEAR AND ACYCLIC POLYNUCLEAR PARENT HYDRIDES
P-21.1 Mononuclear parent hydridesP-21.1 MONONUCLEAR PARENT HYDRIDES
P-21.2 Acyclic polynuclear parent hydrides
P-21.1.1 Mononuclear parent hydrides with standard bonding numbers
P-21.1.1.1 Systematic names
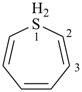
The names of mononuclear hydrides of the elements for use as parents in naming organic compounds by substitutive nomenclature are given in Table 2.1. Most are formed systematically by citing the ‘a’ term of the element (see Table 2.4) with elision of the terminal letter ‘a’ before the ending ‘ane’ (patterned after methane), for example borane for BH3 and silane for SiH4, etc. There are important exceptions: oxidane for H2O, sulfane for H2S, selane for H2Se, tellane for H2Te, polane for H2Po, and bismuthane for BiH3 (see Table 2.1). The systematically formed names oxane, thiane, selenane, tellurane, polonane, and bismane are Hantzsch-Widman names designating the corresponding saturated six-membered rings with a single heteroatom. The name ‘carbane’ has never been used in place of methane; it is not recommended.
| Groups | 13 | 14 | 15 | 16 | 17 |
| BH3 borane | CH4 (carbane)* | NH3 azane | OH2 oxidane | FH fluorane | |
| AlH3 alumane | SiH4 silane | PH3 phosphane | SH2 sulfane | ClH chlorane | |
| GaH3 gallane | GeH4 germane | AsH3 arsane | SeH2 selane | BrH bromane | |
| InH3 indigane | SnH4 stannane | SbH3 stibane | TeH2 tellane | IH iodane | |
| TlH3 thallane | PbH4 plumbane | BiH3 bismuthane | PoH2 polane | AtH astatane |
* Note that methane is the preferred IUPAC name.
See P-21.1.1.2 for common names used in the IUPAC nomenclature of organic and inorganic compounds.

aluminane
InH3
indigane (preselected name, see P-12.2)

indane
2,3-dihydro-1H-indene (PIN)
P-21.1.1.2 Retained names
The common name methane is retained. The common names water, ammonia, the binary names for the hydracids of Group 17, for example hydrogen chloride, and binary names for the hydrides of Group 16, for example hydrogen sulfide, are used in these recommendations but their use as preferred IUPAC names is deferred pending publication of recommendations for the selection of preferred inorganic names; thus, no PIN label will be assigned in names including them (see P-14.8). However, systematic alternatives to these common names, e.g. oxidane for water and azane for ammonia, and for the binary names of hydracids of Group 17 and the hydrides of Group 16, for example chlorane for hydrogen chloride and sulfane for hydrogen sulfide, are necessary for naming some derivatives and for generating names of radicals, ions, and polynuclear homologues.
The names ‘phosphine’ for PH3, ‘arsine’ for AsH3, ‘stibine’ for SbH3, and ‘bismuthine’ for BiH3, are retained for use in general nomenclature.
P-21.1.2 Mononuclear parent hydrides with nonstandard bonding numbers
P-21.1.2.1 Systematic and traditional names
If the bonding number of the element differs from the standard one as defined in P-14.1 and exemplified in Table 2.1, the name of the hydride is modified by affixing the symbol λn, where ‘n’ is the bonding number, to the name of the hydride (ref. 13).
The names ‘phosphorane’ for PH5, ‘arsorane’ for AsH5, and ‘stiborane’ for SbH5, are retained for use in general nomenclature. However, the names ‘sulfurane’ for SH4, ‘selenurane’ for SeH4, ‘iodinane’ for IH3, ‘persulfurane’ for SH6, and ‘periodinane’ for IH5, which have been used in recent literature, are not recommended.
Examples:
SH4
λ4-sulfane (preselected name, see P-12.2)
(not sulfurane)
SnH2
λ2-stannane (preselected name, see P-12.2)
PH5
λ5-phosphane (preselected name, see P-12.2)
phosphorane
AsH5
λ5-arsane (preselected name, see P-12.2)
arsorane
SbH5
λ5-stibane (preselected name, see P-12.2)
stiborane
P-21.2.1 HydrocarbonsP-21.2.1 Hydrocarbons
P-21.2.2 Homogeneous acyclic parent hydrides other than hydrocarbons and boron hydrides
P-21.2.3 Heterogenous acyclic parent hydrides
P-21.2.4 Acyclic parent hydrides containing heteroatoms with nonstandard bonding numbers
The saturated unbranched acyclic hydrocarbons C2H6, C3H8, and C4H10 have the retained names ethane, propane, and butane, respectively. Systematic names for the higher members of this series consist of a numerical term (see Table 1.4) followed by the ending ‘ane’ with elision of the terminal letter ‘a’ from the numerical term. The generic name for saturated acyclic hydrocarbons (branched or unbranched) is ‘alkane’. The chain is numbered from one end to the other with arabic numbers. Brackets are employed in formulas to indicate repetition of groups in chains. For unsaturated acyclic hydrocarbons see P-31.1.
Examples:

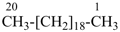
icosane (PIN)
(the Beilstein and CAS index name is ‘eicosane’)
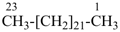
tricosane (PIN)
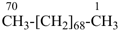
heptacontane (PIN)
A compound consisting of an unbranched chain containing two or more identical heteroatoms saturated with hydrogen atoms is named by citing the appropriate multiplying prefix from Table 1.4 (with no elision of the terminal vowel of the multiplying prefix) followed by the name of the appropriate hydride according to P-21.1. These names are preselected (see P-12.2). Potential functionality of terminal groups, such as –SH or –OH, is ignored.
Examples:
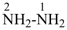
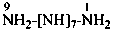
nonaazane (preselected name, see P-12.2)
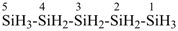
pentasilane (preselected name, see P-12.2)
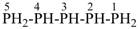
pentaphosphane (preselected name, see P-12.2)
HS-S-S-SH
tetrasulfane (preselected name)
(not disulfanedithiol nor trisulfanethiol, see P-58.3.1)
P-21.2.3.1 Heterogeneous parent hydrides composed of alternating atoms
P-21.2.3.2 Heterogeneous parent hydrides formed by skeletal replacement (‘a’) nomenclature
P-21.2.3.1 Heterogeneous parent hydrides composed of alternating heteroatoms, i.e., [a(ba)n hydrides], excluding carbon atoms and the halogen atoms
Compounds containing an unbranched chain of alternating atoms terminated by two identical atoms of the element coming later in the seniority order O > S > Se > Te > N > P >As > Sb > Bi > Si > Ge > Sn > Pb > B >Al > Ga > In > Tl maybe named by citing successively a multiplying prefix denoting the number of atoms of the terminal element followed by the ‘a’ term for that element, then the ‘a’ term of the other element in the chain and the ending ‘ane’. The terminal letter ‘a’ of an ‘a’ term is elided when followed by a vowel; the terminal vowel of a numerical prefix is not elided even when the ‘a’ term begins with the same vowel. When nitrogen atoms are present, amine names (see P-62) should be used because of the higher functionality of amines. This concept does not extend to other elements. These parent hydrides are preselected parent hydrides (see P-12.2) and have priority to receive preferred IUPAC names, as long as they are used to name compounds containing carbon.
This is a change from the 1993 Guide (ref. 2) where the ‘amine’ characteristic group was not recognized.
Examples:
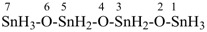
SiH3-S-SiH3
disilathiane (preselected name, see P-12.2)
HS-O-SH
dithioxane (preselected name, see P-12.2)
PH2-Se-PH2
diphosphaselenane (preselected name, see P-12.2)
SiH3-NH-SiH3
N-silylsilanamine (name derived from the preselected name silane, see P-12.2)
(not disilazane)
Heterogeneous acyclic parent hydrides consisting of chains containing at least one carbon atom and heteroatoms, alike or different, and terminating with C, P, As, Sb, Bi, Si, Ge, Sn, Pb, B, Al, Ga, In, or Tl are named by skeletal replacement (‘a’) nomenclature (see P-15.4.3). The use of skeletal replacement (‘a’) names as preferred IUPAC names is considered in Chapter P-5.
Examples:
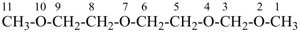
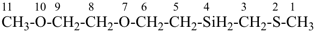
7,10-dioxa-2-thia-4-silaundecane (PIN)
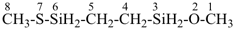
2-oxa-7-thia-3,6-disilaoctane (PIN)
P-21.2.4.1 Heteroatoms with nonstandard bonding numbers are denoted by the λn symbol which is placed after each appropriate locant (see P-14.1). Low numbering is first given to the heteroatoms in the usual manner without regard to nonstandard bonding numbers. When there is a choice, lower locants are given to a higher nonstandard bonding number.
Examples:
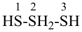
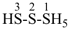
1λ6-trisulfane (preselected name, see P-12.2)
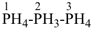
1λ5,2λ5,3λ5-triphosphane (preselected name, see P-12.2)
(not tri-λ5-phosphane)
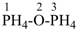
1λ5,3λ5-diphosphoxane (preselected name, see P-12.2)
Examples:
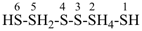
P-22.1 Monocyclic hydrocarbonsP-22.1 MONOCYCLIC HYDROCARBONS
P-22.2 Heteromonocyclic parent hydrides
P-22.1.1 The names of saturated monocyclic hydrocarbons are formed by attaching the nondetachable prefix ‘cyclo’ to the name of the acyclic saturated unbranched hydrocarbon with the same number of carbon atoms. The generic name of monocyclic hydrocarbons is ‘cycloalkane’. Numbering proceeds sequentially around the ring, usually clockwise. For unsaturated monocyclic hydrocarbons, see P-31.1.
Examples:

cyclohexane (PIN)
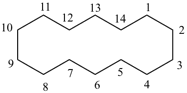
cyclotetradecane (PIN)
Preferred IUPAC names for fully unsaturated monocyclic hydrocarbons are those of the corresponding cycloalkapolyenes (see P-31.1.3.1).
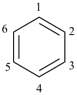
Benzene is the retained name for C6H6; the name [6]annulene is not recommended.
In the numbering of annulenes, the locant ‘1’ is placed at any carbon atom in structures having an even number of carbon atoms; in annulenes having an odd number of carbon atoms, the locant ‘1’ is assigned to the carbon atom bearing the indicated hydrogen (see P-14.7). In cycloalkapolyene structures, the locant ‘1’ is always assigned to a carbon atom of a double bond.
Examples:
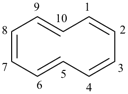
[10]annulene
cyclodeca-1,3,5,7,9-pentaene (PIN, P-25.3.2.1.1)
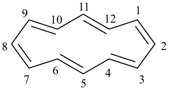
[12]annulene
cyclododeca-1,3,5,7,9,11-hexaene (PIN, P-31.1.3.1)
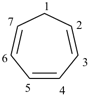 | 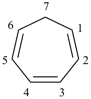 |
| (I) | (II) |
| (I) 1H-[7]annulene (II) cyclohepta-1,3,5-triene (PIN, P-31.1.3.1) | |
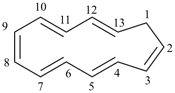 | 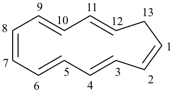 |
| (I) | (II) |
| (I) 1H-[13]annulene (II) cyclotrideca-1,3,5,7,9,11-hexaene (PIN, P-31.1.3.1) | |
Toluene, xylene, and mesitylene are specific parent hydrides that are composed of two components, one cyclic and the other acyclic and saturated. These names are retained due to a long and well established tradition. Toluene and xylene are preferred IUPAC names, but are not freely substitutable; toluene is substitutable under certain conditions, but only for general nomenclature (see P-15.1.8 for a general substitution rules for retained names).
Mesitylene is a retained name in general nomenclature only and cannot be substituted.
The names cumene and cymene are not retained.
In the 1993 Guide (ref. 2), these parent hydrides were retained but only limited substitution was allowed.
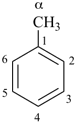 | toluene (PIN) (no substitution; for general nomenclature substitution is permitted only by groups listed in P-15.1.8.2.2) |
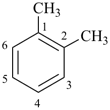 | xylene (1,2-, 1,3-, and 1,4-isomers, PINs) (no substitution) o-, m-, and p-xylene |
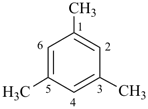 | mesitylene (no substitution) 1,3,5-trimethylbenzene (PIN) |
P-22.2.1 Retained names of heteromonocyclesP-22.2.1 Retained names of heteromonocycles
P-22.2.2 Heteromonocyclic parent hydrides with 3-10 membered rings (Hantzsch-Widman names)
P-22.2.3 Heteromonocyclic parent hydrides named by skeletal replacement (‘a’) nomenclature
P-22.2.4 Heteromonocycles with eleven or more members with the maximum number of noncumulative double bonds
P-22.2.5 Homogeneous monocyclic parent hydrides other than boron hydrides
P-22.2.6 Heteromonocyclic parent hydrides composed of repeating heterounits
P-22.2.7 Heteromonocyclic parent hydrides having heteroatoms with nonstandard bonding numbers
Retained names for ‘mancude’ heteromonocycles and their chalcogen analogues are listed in Table 2.2.
Note: The term ‘mancude’ is an acronym for MAximum number of NonCUmulative DoublE bonds.The name ‘pyran’ is modified by functional replacement nomenclature to generate names for chalcogen analogues.
Retained names for saturated heteromonocycles are given in Table 2.3.
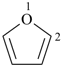
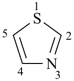
furan (PIN)
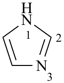
imidazole (1H-isomer shown;
the PIN is 1H-imidazole)
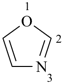 | 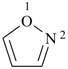 |
| oxazole 1,3-oxazole (PIN) thiazole (S instead of O) selenazole (Se instead of O) tellurazole (Te instead of O) | isoxazole 1,2-oxazole (PIN) isothiazole (S instead of O) isoselenazole (Se instead of O) 1,2-selenazole (PIN) isotellurazole (Te instead of O) |
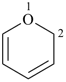
pyran (2H-isomer shown;
the PIN is 2H-pyran)
thiopyran (S instead of O) (2H-isomer shown;
the PIN is 2H-thiopyran)
selenopyran (Se instead of O) (2H-isomer shown;
the PIN is 2H-selenopyran)
telluropyran (Te instead of O) (2H-isomer shown;
the PIN is 2H-telluropyran)
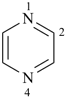
pyrazine (PIN)
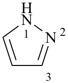
pyrazole (1H-isomer shown;
the PIN is 1H-pyrazole)
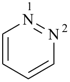
pyridazine (PIN)
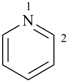
pyridine (PIN)
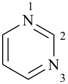
pyrimidine (PIN)
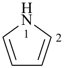
pyrrole (1H-isomer shown;
the PIN is 1H-pyrrole)
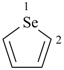
selenophene (PIN)
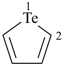
tellurophene (PIN)
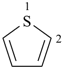
thiophene (PIN)
Table 2.3 Retained names of saturated heteromonocyclic parent hydrides
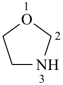 | 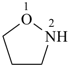 |
| oxazolidine 1,3-oxazolidine (PIN) thiazolidine (S instead of O) selenazolidine (Se instead of O) tellurazolidine (Te instead of O) | isoxazolidine 1,2-oxazolidine (PIN) isothiazolidine (S instead of O) 1,2-thiazolidine (PIN) isoselenazolidine (Se instead of O) isotellurazolidine (Te instead of O) |
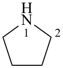
pyrrolidine (PIN)
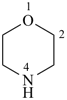
morpholine (PIN)
thiomorpholine (S instead of O) (PIN)
selenomorpholine (Se instead of O) (PIN)
telluromorpholine (Te instead of O) (PIN)
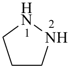
pyrazolidine (PIN)
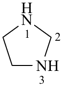
imidazolidine (PIN)
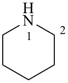
piperidine (PIN)
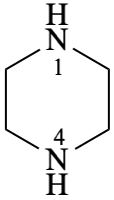
piperazine (PIN)
Heteromonocyclic parent hydrides with no more than ten ring members and containing one or more heteroatoms are named by using the extended Hantzsch-Widman system (ref. 21), a modification of the method published in the 1979 recommendations (ref. 1) that removes the need for many of the explanatory footnotes and comments. Homogeneous heteromonocyclic ring names are preselected (see P-12.2). The recommendations in the 1979 recommendations continue to be used in CAS fused ring names.
The elements aluminium, gallium, indium, and thallium are now included in the recommended Hantzsch-Widman system and mercury has been deleted.
P-22.2.2.1 Constructing and numbering Hantzsch-Widman names
P-22.2.2.1.1 Hantzsch-Widman names are formed by combining skeletal replacement (‘a’) prefix(es) for the heteroatom(s) (Table 2.4) with a stem indicating the size of the ring and the degree of hydrogenation (Table 2.5). The final ‘a’ vowel between ‘a’ prefixes and between the ‘a’ prefix and the stem are elided. Unsaturated compounds are those having the maximum number of noncumulative double bonds (mancude compounds) and at least one double bond. The presence of a single heteroatom determines the numbering in a monocyclic compound; the heteroatom has the locant ‘1’ and the numbering usually proceeds clockwise, when unsubstituted.
Hantzsch-Widman names, except for azine and oxine, are preferred IUPAC names for both the unsaturated and saturated compounds. Azine must not be used for pyridine because of its long-established use as a class name for =N-N= compounds (see P-68.3.1.2.3). Oxine must not be used for pyran because it has been used as a trivial name for quinolin-8-ol.
Hantzsch-Widman names are preselected names for homogeneous heteromonocycles other than hydrocarbons (see P-22.2.5).
The final ‘e’ in Hantzsch-Widman names is required in preferred IUPAC names; it is still optional in general nomenclature. In the 1979 Rules (ref. 1), the final ‘e’ of a Hantzsch-Widman name was omitted when there was no nitrogen in the ring; in the 1993 Guide (ref. 2) this omission was made optional.
Examples:
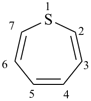
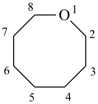
oxocane (PIN)
Table 2.4 Hantzsch-Widman system prefixes (in decreasing order of seniority)
| Element | Bonding Number (Valence) | Prefix | Element | Bonding Number (Valence) | Prefix | |
| fluorine | 1 | fluora | antimony | 3 | stiba | |
| chlorine | 1 | chlora | bismuth | 3 | bisma | |
| bromine | 1 | broma | silicon | 4 | sila | |
| iodine | 1 | ioda | germanium | 4 | germa | |
| oxygen | 2 | oxa | tin | 4 | stanna | |
| sulfur | 2 | thia | lead | 4 | plumba | |
| selenium | 2 | selena | boron | 3 | bora | |
| tellurium | 2 | tellura | aluminium | 3 | aluma1 (not alumina) | |
| nitrogen | 3 | aza | gallium | 3 | galla | |
| phosphorus | 3 | phospha | indium | 3 | indiga1 (not inda) | |
| arsenic | 3 | arsa | thallium | 3 | thalla |
1 Compare with Table 1.5
Table 2.5 Hantzsch-Widman system stems
| Ring Size | Unsaturated | Saturated | Ring Size | Unsaturated | Saturated | |
| 3 | irene/irine1 | irane/iridine2 | 7 | epine | epane | |
| 4 | ete | etane/etidine2 | 8 | ocine | ocane | |
| 5 | ole | olane/olidine2 | 9 | onine | onane | |
| 6A (O, S ,Se, Te, Bi) | ine | ane | 10 | ecine | ecane | |
| 6B (N, Si, Ge, Sn, Pb) | ine | inane | ||||
| 6C (F, Cl, Br, I, P, As, Sb, B, Al, Ga, In, Tl) | inine | inane | ||||
| 1 See P-22.2.2.1.5.1 | ||||||
| 2 See P-22.2.2.1.5.2 | ||||||
P-22.2.2.1.2 A multiplicity of the same heteroatom is indicated by a multiplying prefix ‘di’, ‘tri’, ‘tetra’, etc., placed before the appropriate ‘a’ term. The final letter ‘a’ of a multiplying prefix is elided before a vowel, e.g., tetrazole, not tetraazole. Lowest possible locants are assigned to heteroatoms, locant ‘1’ being assigned to one of the heteroatoms. Locants are cited at the front of the name, i.e., before the skeletal replacement (‘a’) term and any preceding numerical prefixes.
Examples:
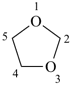
1,3-dioxolane (PIN)
Examples:
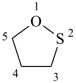
1,2-oxathiolane (PIN)
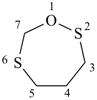
1,2,6-oxadithiepane (PIN)
(not 1,3,7-oxadithiepane;
the locant set ‘1,2,6’ is lower than ‘1,3,7’)
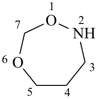
1,6,2-dioxazepane (PIN)
(not 1,3,4-dioxazepane;
not 1,3,7-dioxazepane;
not 1,6,5-dioxazepane;
the locant set ‘1,2,6’ is lower than ‘1,3,4’, ‘1,3,7’, or ‘1,5,6’)
Example:
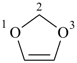
After the maximum number of noncumulative double bonds has been assigned to the ring structure, any ring atom with a bonding number of three or higher connected to adjacent ring atoms by single bonds only, and carrying one or more hydrogen atoms, is designated by indicated hydrogen (see P-14.7). If there is a choice, such ring atoms are assigned low locants.
Examples:
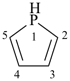
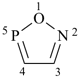
1H-phosphole (PIN)
2H-1,3-dioxole (PIN)
As shown in Table 2.5, for mancude three-membered rings and saturated three-, four-, and five-membered rings two stems are recommended. They are used as follows to generate preferred IUPAC names:
P-22.2.2.1.5.1 The stem ‘irine’ is used in place of ‘irene’ for rings only containing nitrogen heteroatoms; otherwise the stem ‘irene’ is used.
Examples:
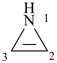
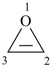
oxirene (PIN)
oxazirene (PIN)
Examples:
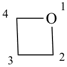
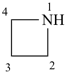
azetidine (PIN)
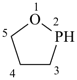
1,2-oxaphospholane (PIN)
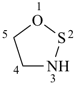
1,2,3-oxathiazolidine (PIN)
The stem for six-membered rings depends on the least senior heteroatom in the ring, i.e., the heteroatom whose name directly precedes the stem. Heteroatoms are divided into three groups, A, B, and C, each corresponding to a stem for the unsaturated and for the saturated compound (Table 2.5). The stem is selected in accordance with the group to which the least senior heteroatom belongs.
Examples:
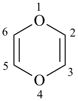
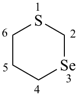
1,3-thiaselenane (PIN)
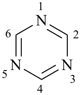
1,3,5-triazine (PIN)
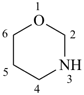
1,3-oxazinane (PIN)
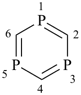
1,3,5-triphosphinine (PIN)
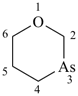
1,3-oxarsinane (PIN)
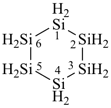
hexasilinane (preselected name; see P-12.2)
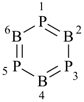
1,3,5,2,4,6-triphosphatriborinine
( preselected name; see P-12.2)
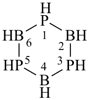
1,3,5,2,4,6-triphosphatriborinane (preselected name; see P-12.2)
All locants are omitted for parent Hantzsch-Widman names if there is only one heteroatom or if there is no ambiguity if locants are omitted.
Examples:
Mancude and saturated heteromonocyclic compounds with up to and including ten ring members are named by the extended Hantzsch-Widman system (see P-22.2.2). For monocyclic rings with eleven and more ring members, skeletal replacement (‘a’) nomenclature (see P-15.4) is used for the fully saturated or fully unsaturated compounds ([n]annulenes).
P-22.2.3.1 Skeletal replacement (‘a’) names are formed by placing skeletal replacement (‘a’) prefixes (see Table 2.4) in front of the name of the corresponding cycloalkane or annulene, and, when more than one heteroatom is present, in the following decreasing seniority order: F > Cl > Br > I > O > S > Se > Te > N > P > As > Sb > Bi > Si > Ge > Sn > Pb > B > Al > Ga > In > Tl. For numbering, see P-22.2.3.2. The selection of preferred IUPAC names is discussed in P-52.2.3.
Examples:
1-azacyclotetradeca-1,3,5,7,9,11,13-heptaene (PIN)
aza[14]annulene
P-22.2.3.2.1 When a single heteroatom is present in the ring, it is assigned the locant ‘1’, which is omitted in the name, unless a locant for an indicated hydrogen atom is present. Numbering usually proceeds clockwise, unless substituted. Low locants are assigned first to the heteroatom and then to unsaturated sites (see P-31.1.3.2). When required, locants for indicated hydrogen atoms are assigned in accordance with P-14.7
. Examples:
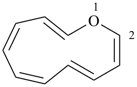
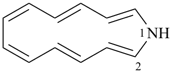
1-azacyclotrideca-2,4,6,8,10,12-hexaene (PIN)
1H-1-aza[13]annulene
Examples:
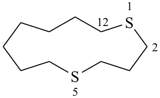
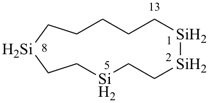
1,2,5,8-tetrasilacyclotridecane (PIN)
(other locant sets are: ‘1,2,8,11’, ‘1,4,7,8’, ‘1,4,5,11’, and ‘1,4,10,11’,
none of which are lower than ‘1,2,5,8’)
Examples:
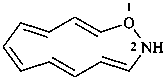
1-oxa-2-azacyclododeca-3,5,7,9,11-pentaene (PIN)
2H-1-oxa-2-aza[12]annulene
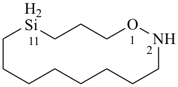
1-oxa-2-aza-11-silacyclotetradecane (PIN)
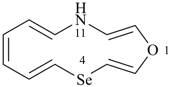
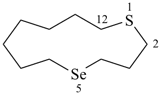
1-oxa-4-selena-11-azacyclotrideca-2,5,7,9,12-pentaene (PIN)
11H-1-oxa-4-selena-11-aza[13]annulene
Names of heteromonocyclic parent components with more than ten ring members are discussed in this subsection. They are used to generate names of preferred IUPAC names of fused ring systems; they have also been used as names for the heteromonocycle in general nomenclature as an alternative to cycloalkapolyene names that are preferred IUPAC names for the heteromonocycles.
A heteromonocyclic parent component having more than ten ring members and the maximum number of noncumulative double bonds (mancude) is named by changing the ending ‘ane’ of the name corresponding to the saturated heteromonocycle (see P-22.2.3) to ‘ine’. Locants are cited at the front of the name, in the order of citation of the corresponding skeletal replacement (‘a’) prefixes. Indicated hydrogen atoms are designated as required. In organometallic nomenclature, a modified skeletal replacement (‘a’) nomenclature is allowed in smaller rings to create metallacycle parent hydrides (see P-69.4).
For examples of fusion compounds including this type of heteromonocyclic component, see P-25.2.2.4, P-25.3.6.1 and P-25.3.7.1.
Examples:
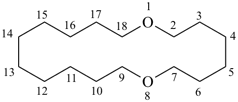
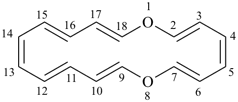
1,8-dioxacyclooctadecine
1,8-dioxacyclooctadeca-2,4,6,9,11,13,15,17-octaene (PIN)
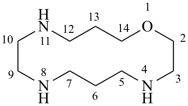
1-oxa-4,8,11-triazacyclotetradecane (PIN)
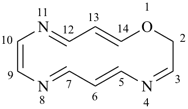
2H-1,4,8,11-oxatriazacyclotetradecine
1-oxa-4,8,11-triazacyclotetradeca-3,5,7,9,11,13-hexaene (PIN)
A saturated heteromonocycle consisting of identical heteroatoms can be named by adding the prefix ‘cyclo’ to the name of the saturated unbranched chain that has the same number of identical atoms. For alternative methods, see the Hantzsch-Widman extended system described in P-22.2.2 for rings with 3 through 10 members and skeletal replacement (‘a’) nomenclature described in P-22.2.3. For the names that are preselected (see P-12.2) but used to generate preferred IUPAC names for organic derivatives, see P-52.1.5.
Examples:
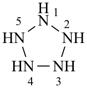
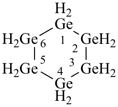
cyclohexagermane
hexagerminane
(preselected name; see P-12.2)
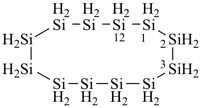
cyclododecasilane
dodecasilacyclododecane
(preselected name; see P-12.2)
Names may be constructed by citing successively the prefix ‘cyclo’, a multiplying affix (‘di’, ‘tri’, ‘tetra’, etc.) indicating the number of each element in the ring, the skeletal replacement (‘a’) terms for the atoms of the repeated unit first cited in the order Tl > In > Ga > Al > Pb > Sn > Ge > Si > Bi > Sb> As > P > N > Te > Se > S> O, and the ending ‘ane’. The terminal letter of a skeletal replacement (‘a’) term is elided when followed by a vowel; the terminal letter of a multiplying affix is not elided even when the ‘a’ term begins with a vowel. Numbering starts at one of the skeletal atoms of the element cited last in the name and proceeds continuously around the ring. For alternative methods, see P-22.2.2 for Hantzsch-Widman names and P-22.2.3 for monocycles with more than ten ring members. For the preselected names (see P-12.2) used to generate the preferred IUPAC names for organic derivatives of these heteromonocyclic hydrides, see P-52.1.5.2.
Examples:
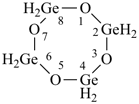
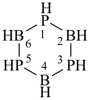
cyclotriboraphosphane
1,3,5,2,4,6-triphosphatriborinane
(preselected name, see P-12.2)
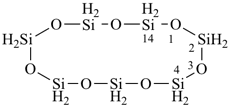
cycloheptasiloxane
1,3,5,7,9,11,13-heptaoxa-2,4,6,8,10,12,14-heptasilacyclotetradecane
(preselected name, see P-12.2, P-22.2.3.2.3)
P-22.2.7.1 The λ-convention is used to denote heteroatoms with nonstandard bonding numbers in heteromonocycles (see P-14.1). The symbol λn, where n is the bonding number, is cited immediately after the locant denoting the heteroatom with the nonstandard bonding number. The indicated hydrogen symbol H, if required to denote saturated skeletal atoms, is cited at the front of the complete name with the appropriate locant(s).
Examples:
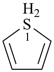
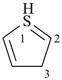
3H-1λ4-thiophene (PIN)
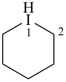
1λ3-iodinane (PIN)
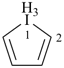
1H-1λ5-iodole (PIN)
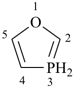
1,3λ5-oxaphosphole (PIN)
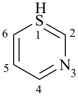
1λ4,3-thiazine (PIN)
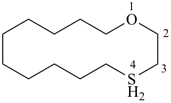
1-oxa-4λ4-thiacyclotetradecane (PIN)
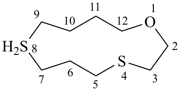
1-oxa-4,8λ4-dithiacyclododecane (PIN)

1H-1λ4-thiepine (PIN)
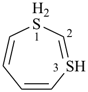
1H-1λ4,3λ4-dithiepine (PIN)
[the (–SH2–) group, which includes an indicated hydrogen, is preferred for the lowest locant]
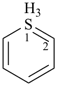
1λ6-thiopyran (PIN)
(this heteromonocycle has the maximum number of double bonds and one double bond at every position;
hence, no indicated hydrogen is cited for the sulfur atom)
Examples:
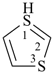
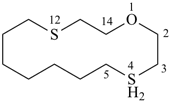
1-oxa-4λ4,12-dithiacyclotetradecane (PIN)
P-23.0 IntroductionP-23.0 INTRODUCTION
P-23.1 Definitions and terminology
P-23.2 Naming and numbering of von Baeyer hydrocarbons
P-23.3 Heterogeneous heterocyclic von Baeyer parent hydrides
P-23.4 Homogeneous heterocyclic von Baeyer parent hydrides
P-23.5 Heterogeneous heterocyclic von Baeyer parent hydrides composed of alternating heteroatoms
P-23.6 Heterocyclic polyalicyclic parent hydrides having heteroatoms with nonstandard bonding numbers
P-23.7 Retained names for von Baeyer parent hydrides
This section is based on the recent publication “Extension and Revision of the von Baeyer system for naming polycyclic compounds (including bicyclic compounds) (IUPAC Recommendations 1999)” (ref. 7). It supersedes Rules A-31, A-32, and B-14 in the 1979 Recommendations (ref. 1) and Rule R-2.4.2 in the 1993 Recommendations (ref. 2). No modifications to the 1999 publication have been made in this Section.
This Section deals only with saturated polyalicyclic ring systems named by the von Baeyer system; for unsaturated systems, see Section P-31.1.4. For naming substituent groups derived from saturated polyalicyclic ring systems, see Section P-29.
P-23.1 DEFINITIONS AND TERMINOLOGY
P-23.1.1 A ‘bridgehead’ is any skeletal atom of the ring system that is bonded to three or more skeletal atoms (excluding hydrogen).
P-23.1.2 A ‘bridge’ is an unbranched chain of atoms or an atom or a valence bond connecting two bridgeheads.
P-23.1.3 The ‘main ring’ is the ring system that includes as many skeletal atoms of the polycyclic system as possible.
P-23.1.4 The ‘main bridge’ is the bridge included in a bicyclic system and the first selected bridge in a polycyclic system.
P-23.1.5 Two bridgeheads are selected as ‘main bridgeheads’. These two bridgeheads are included in the main ring and connected by the main bridge.
P-23.1.6 A ‘secondary bridge’ is any bridge not included in the main ring or the main bridge.
P-23.1.7 An ‘independent secondary bridge’ links bridgeheads which are part of the main ring or main bridge.
P-23.1.8 A ‘dependent secondary bridge’ links at least one bridgehead that is part of a secondary bridge.
P-23.1.9 A ‘polycyclic system’ contains a number of rings equal to the minimum number of scissions required to convert the system into an acyclic skeleton. The number of rings is indicated by the nondetachable prefix ‘bicyclo’ (not dicyclo), ‘tricyclo’, ‘tetracyclo’, etc.
P-23.2 NAMING AND NUMBERING OF VON BAEYER HYDROCARBONS
Bi- and polycyclic hydrocarbons that are treated by the von Baeyer system are named by the following rules applied in order until a decision is reached.
P-23.2.1 Selection of the main ringP-23.2.1 Selection of the main ring
P-23.2.2 Naming bicyclic alicyclic hydrocarbons
P-23.2.3 Numbering bicyclic alicyclic hydrocarbons
P-23.2.4 Selection of the main bridge
P-23.2.5 Naming and numbering tricyclic alicyclic hydrocarbons
P-23.2.6 Naming and numbering polyalicyclic hydrocarbons
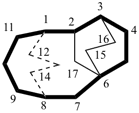
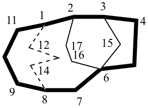
The main ring of a polycyclic hydrocarbon ring system is selected so as to include as many skeletal atoms of the structure as possible. The main ring is shown as bold lines in subsections P-23.2.1 through P-23.2.6.
Examples:
a seven-membered main ring
Saturated homogeneous bicyclic hydrocarbons having two or more atoms in common are named by prefixing ‘bicyclo’ to the name of the acyclic hydrocarbon having the same total number of skeletal atoms. The numbers of skeletal atoms in each of the two segments connecting the main bridgeheads and in the main bridge are given by arabic numbers cited in descending numerical order, separated by full stops, and enclosed in square brackets.
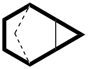
Example:
The bicyclic ring system is numbered starting with one of the bridgeheads and proceeding first along the longer segment of the main ring to the second bridgehead, then back to the first bridgehead along the unnumbered segment of the main ring. Numbering is completed by numbering the main bridge beginning with the atom next to the first bridgehead.
Examples:

bicyclo[4.4.2]dodecane (PIN)
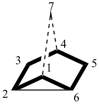
In a polycyclic ring system there is more than one bridge connecting atoms of the main ring and/or the main bridge. In the tricyclic ring below, the main ring is shown in solid bold lines, and the main bridge, shown in the dashed lines, is the bridge that includes as many of the atoms as possible that are not included in the main ring. Bridges other than the main bridge are called ‘secondary bridges’, appearing below as a normal solid line.
Example:
P-23.2.5.1 Tricyclic hydrocarbons having an independent secondary bridge are named on the basis of a bicyclic system described in P-23.2.2. Rings not described by the bicyclic system are defined by citing the number of atoms in the independent secondary bridge as an arabic number. The locants of the two attachment points of the independent secondary bridge to the main ring are cited as a pair of superscript arabic numbers (lower number is cited first) separated by a comma.
The name of the tricyclic system is then constructed by citing:
(a) the prefix ‘tricyclo’, in place of ‘bicyclo’, indicating the presence of three rings in the polyalicyclic system;Example:(b) numbers indicating the bridge lengths, starting with the two branches of the main ring (shown as a bold line in the structures below), followed by the main bridge (shown in dotted bold lines in the structures below), and the secondary bridge (with superscript locants separated by commas indicating its points of attachment to the main ring), all separated by full stops and placed in brackets, for example [2.2.1.02,6];
(c) the name of the acyclic hydrocarbon having the same total number of skeletal atoms.
After the main ring and main bridge have been numbered, the independent secondary bridge is numbered continuing from the higher numbered bridgehead of the main ring.
Examples:
tricyclo[4.2.2.22,5]dodecane (PIN)
[the secondary bridge is numbered starting from bridgehead 5, the higher (than 2) numbered bridgehead]
Polycyclic analogues of saturated bi- and tricyclic ring systems (P-23.2.3 and P-23.2.5) are named as described in the following subsections. Independent and dependent secondary bridges are considered here. Rules for numbering all secondary bridges and for naming all polyalicyclic systems are described; their application follows those described for naming and numbering bicyclic systems as described in P-23.2.1 through P-23.2.4 above. An additional rule is necessary to select the main bridge and the secondary bridges.
P-23.2.6.1 Naming polycyclic alicyclic hydrocarbons
Rings not designated by the bicyclic system described above (P-23.2.2) are defined by citing the number of atoms in each secondary bridge as an arabic number. The locants of the two attachment points of each secondary bridge to the main ring are cited as a pair of superscript arabic numbers (lower first) separated by a comma. The numbers indicating independent secondary bridges (bridges that connect atoms of the bicyclic system) are cited in decreasing order. The procedure for construction of names is given in the following subsections.
P-23.2.6.1.1 The prefixes ‘tricyclo’, ‘tetracyclo’, etc., in place of vbicyclo’, indicate the number of rings in the polyalicyclic system. The number of rings is equal to the number of bond cuts necessary to transform the polycyclic system into an acyclic skeleton, unbranched or branched.
P-23.2.6.1.2 The number of atoms in each bridge additional to the main bridge, i.e., the secondary bridges, is indicated by arabic numbers separated by full stops and cited in decreasing numerical order following those describing the bicyclic system, except as provided by P-23.2.6.1.3. The location of each secondary bridge is indicated by the arabic number locants of the bicyclic structure already numbered; these locants are cited as superscripts to the arabic number denoting its length (number of atoms) and separated by a comma. The assemblage of arabic numbers denoting the length of bridges with superscript numbers, if necessary, is commonly called the ‘von Baeyer descriptor’ and is enclosed in brackets.
P-23.2.6.1.3 Independent secondary bridges are cited before dependent secondary bridges. The numbers indicating dependent secondary bridges are cited in decreasing order (the third example in P-23.2.6.3 illustrates this order of citation).
P-23.2.6.1.4 The name is terminated by the name of the alkane representing the total number of ring atoms; this number corresponds to the sum of the arabic numbers in the numerical descriptor enclosed by brackets plus two (for the two main bridgehead atoms); for example, in the name ‘bicyclo[2.2.1.02,6]heptane’ for the following structure, the total number of ring atoms, 7, equals the sum of the primary numbers in the descriptor, [2 + 2 + 1+ 0], + 2.

P-23.2.6.2 Selection of the main bridge and secondary bridges
There are often a number of choices to be made in the selection of the main bridge and the secondary bridges. To make such choices, the following criteria are applied in order until a decision can be made.
Note: Numberings shown in the examples below follow the rules given in P-23.2.6.3.P-23.2.6.2.1 The main ring must be divided as symmetrically as possible by the main bridge, which, as directed in P-23.2.4, includes as many of the atoms not included in the main ring as possible.
Example:
 | not |  |
| tricyclo[4.3.1.12,5]undecane (PIN) correct | tricyclo[5.2.1.12,6]undecane incorrect |
P-23.2.6.2.2 If there is a choice for independent secondary bridges, the first cited must be as long as possible. Then, if relevant, the second must be as long as possible, etc.
Example:
 | not |  |
| tetracyclo[8.6.6.52,9.123,26]octacosane (PIN) correct | tetracyclo[8.6.6.42,9.223,25]octacosane incorrect |
P-23.2.6.2.3 The number of dependent secondary bridges is to be kept to a minimum.
Example:
 | not |  |
| tetracyclo[5.3.2.12,4.03,6]tridecane (PIN) correct | tetracyclo[5.3.2.12,4.06,13]tridecane incorrect |
P-23.2.6.2.4 The superscript locants for the secondary bridges must be as low as possible when considered as a set in ascending numerical order, the decision being made at the first point of difference.
Examples:
 | not | 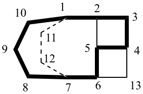 |
| tetracyclo[5.3.2.12,4.03,6]tridecane (PIN) correct | tetracyclo[5.3.2.14,6.02,5]tridecane incorrect |
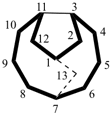 | not | 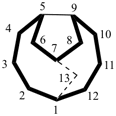 |
| tricyclo[5.5.1.03,11]tridecane correct | tricyclo[5.5.1.05,9]tridecane incorrect |
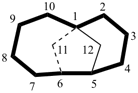 | not | 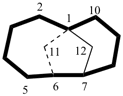 |
| tricyclo[4.4.1.11,5]dodecane (PIN) correct | tricyclo[4.4.1.11,7]dodecane incorrect |
P-23.2.6.2.5 The superscript locants shall be as low as possible when considered in the sequence of their order of citation in the name.
Examples:
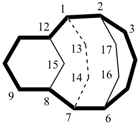 | not | 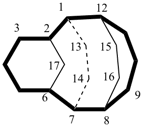 |
| tetracyclo[5.5.2.22,6.18,12]heptadecane (PIN) correct | tetracyclo[5.5.2.28,12.12,6]heptadecane incorrect |
 | not |  |
| pentacyclo[3.3.0.02,4.03,7.06,8]octane (PIN) correct | pentacyclo[3.3.0.02,8.03,7.04,6]octane incorrect |
P-23.2.6.3 Numbering of secondary bridges
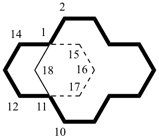
After numbering the main ring and main bridge, independent secondary bridges are numbered before dependent secondary bridges; the numbering continues from the highest number of the main ring and main bridge. Each secondary bridge is numbered in turn starting with the independent secondary bridge linked to the highest numbered bridgehead atom of the main ring, then the independent secondary bridge linked to the next highest bridgehead atom, and so on. Each atom of a secondary bridge is numbered starting with the atom next to the higher numbered bridgehead.
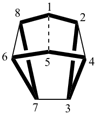
Note: Rule A-32.23 in the 1979 Recommendations (ref. 1) and Rule R-2.4.2.2 in the l993 Recommendations (ref. 2) have been replaced by this Rule.Examples:
 | not |  |
| tetracyclo[4.4.2.22,5.27,10]hexadecane (PIN) | ||
 | not | 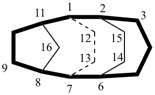 |
| tetracyclo[5.4.2.22,6.18,11hexadecane (PIN) (the first secondary bridged to be numbered is linked to bridgehead 11) | ||
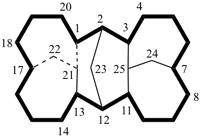
hexacyclo[15.3.2.23,7.12,12.013,21.011,25]pentacosane
(the dependent secondary bridge between atoms numbered 11 and 25 is cited last;
in the numeric descriptor, the numbers ‘15, 3, and 2’ describe the basic bicyclic system;
the numbers 23,7,12,12, and 013,21 correspond to the three independent secondary bridges;
the number 011,25 corresponds to the dependent secondary bridge)
octadecahydro-6,13-methano-2,14:7,9-dipropanodicyclohepta[a,e][8]annulene (PIN) (see P-25.4.3.4.2)
[not octadecahydro-7,14-methano-4,6:8,10-dipropanodicyclohepta[a,d][8]annulene;
in the correct name a greater number of rings of the fusion ring system to be bridged are in a horizontal row,
3 versus 2; see P-25.3.2.3.3 (a) and P-44.2.2.2.3 (b)]
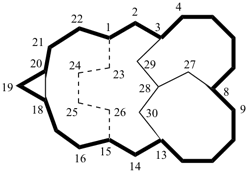 | not | 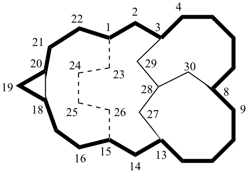 |
| (I) correct | (II) incorrect | |
| (I) pentacyclo[13.7.4.33,8.018,20.113,28]triacontane (PIN) (II) pentacyclo[13.7.4.33,13.018,20.18,28]triacontane | ||
Explanation: In the correct name (I), the PIN, the independent bridge is numbered before the dependent bridge; locant sequence ‘3,8,18,20,13,28’ is lower than ‘3,13,18,20,8,28’ (see P-14.3.5).
P-23.2.6.4 If there is a further choice for numbering the secondary bridges, the following criteria are considered in order until a decision can be made.
P-23.2.6.4.1 Lower locants are used for the atoms of the bridge linked to the higher numbered bridgehead.
Examples:
tetracyclo[6.3.3.22,6.13,6]heptadecane (PIN)
(the locant 15 is assigned to the bridge linked to bridgehead atom 3 not 2)
Example:
P-23.3.1 The only general method to name heterogeneous heterocyclic von Baeyer systems is skeletal replacement (‘a’) nomenclature. The replacement (‘a’) prefixes denoting the heteroatoms are placed in front of the name of the corresponding hydrocarbon named according to P-23.2, cited in the order: F > Cl > Br > I > O > S > Se > Te > N > P > As > Sb > Bi >Si > Ge > Sn > Pb > B > Al > Ga > In > Tl. Numbering is determined first by the fixed numbering of the hydrocarbon system.
Examples:
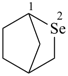
2-selenabicyclo[2.2.1]heptane (PIN)
P-23.3.2.1 Low locants are assigned to the heteroatoms considered together as a set compared in increasing numerical order. The preferred numbering is the lowest set at the first point of difference.
Example:
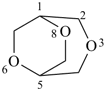 | not | 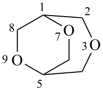 |
| 3,6,8-trioxabicyclo[3.2.2]nonane (PIN) correct | 3,7,9-trioxabicyclo[3.2.2]nonane incorrect |
P-23.3.2.2 If there is still a choice, low locants are assigned in accord with the decreasing seniority order of heteroatoms O > S > Se > Te > N > P > As > Sb > Bi > Si > Ge > Sn > Pb > B > Al > Ga > In > Tl.
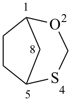
Example:
Heterocyclic von Baeyer systems composed entirely of the same heteroatom are named:
(a) as described for bi- and polycyclic hydrocarbons in P-23.2 using the name of the acyclic parent hydride that has the same total number of skeletal atoms; orIn either method it is not necessary to give the location of the heteroatoms because all positions are modified by the same heteroatom. For the preselected names (see P-12.2) that are used to generate PIN names for organic derivatives see P-52.1.5.(b) by skeletal replacement nomenclature using the replacement (‘a’) prefixes described in P-22.2.5, in which the total number of heteroatoms is indicated by a numerical term.
Examples:
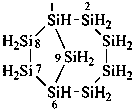
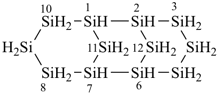
(a) tricyclo[5.3.1.12,6]dodecasilane (preselected name; see P-12.2)
(b) dodecasilatricyclo[5.3.1.12,6]dodecane
P-23.5.1 Heterogeneous von Baeyer systems composed of alternating skeletal heteroatoms may be named in two ways:
(a) by citing a nondetachable prefix ‘bicyclo’, ‘tricyclo’, etc. before a von Baeyer descriptor (see P-23.2.2, P-23.2.5, and P-23.2.6) enclosed in square brackets and then, successively:For the preselected names (see P-12.2) that are used to generate PIN names for organic derivatives see P-52.1.6.2.
(i) a multiplying prefix, ‘di’, ‘tri’, etc., denoting the number of heteroatoms that are first cited as replacement (‘a’) terms;Numbering is the same as for the corresponding hydrocarbon (see P-23.2.3 and P-23.2.6.3);(ii) the sketetal replacement (‘a’) terms denoting the heteroatoms of the system in the reverse order of the recommended seniority for ‘a’ prefixes (for example, Si before O; see P-23.3.1);
(iii) the ending ‘ane’.
(b) by applying normal skeletal replacement (‘a’) nomenclature to the corresponding hydrocarbon.
Examples:
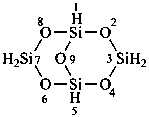
(a) tricyclo[5.1.1.13,5]tetrasilathiane (preselected name; see P-12.2)
(b) 2,4,6,8,9,10-hexathia-1,3,5,7-tetrasilatricyclo[5.1.1.13,5]decane
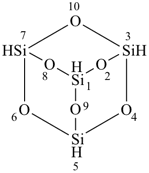
(a) tricyclo[3.3.1.13,7]tetrasiloxane (preselected name; see P-12.2)
(b) 2,4,6,8,9,10-hexaoxa-1,3,5,7-tetrasilatricyclo[3.3.1.13,7]decane
Examples:
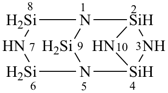
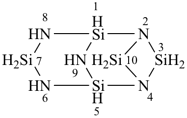
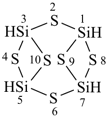
(a) 1Si-tricyclo[3.3.1.12,4]pentasilazane (preselected name; see P-12.2)
(b) 2,4,6,8,9-pentaaza-1,3,5,7,10-pentasilatricyclo[3.3.1.12,4]decane
Compounds in which each silicon atom is linked to three oxygen atoms and in which every atom of oxygen is linked to two silicon atoms are named generically silasesquioxanes. Similarly, when the oxygen atoms are replaced by S, Se, Te, or N atoms, the compounds are generically called silasesquithianes, silasesquiazanes, and so forth. They are named by the method described in P-23.5.1 (a). Silasesquioxanes have the general formula Si2nH2nO3n. The names tetrasilasesquioxanes (n = 2), hexasilasesquioxanes (n = 3), etc., are class names indicating Si2nH2nE3n where E = O; and similarly when E = S, Se, Te, or N. Silasesquiazanes have the general formula Si2nH5nN3n.
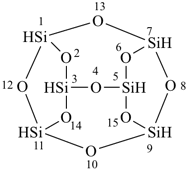
P-23.6.1 The λ-convention, characterized by the symbol λn, where ‘n’ is the bonding number of the heteroatom, is used to identify heteroatoms with nonstandard bonding numbers (see P-14.1). The symbol is placed before the appropriate ‘a’ prefix.
Example:
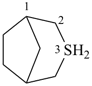
Example:
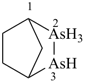
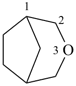
The retained names adamantane and cubane are used in general nomenclature and as preferred IUPAC names. The name quinuclidine is retained for general nomenclature only (see Table 2.6). The name prismane is no longer recommended.
adamantane (PIN)
tricyclo[3.3.1.13,7]decane
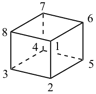
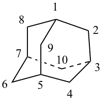
cubane (PIN)
pentacyclo[4.2.0.02,5.03,8.04,7]octane
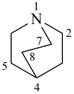
quinuclidine
1-azabicyclo[2.2.2]octane (PIN)
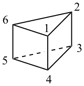
tetracyclo[2.2.0.02,6.03,5]hexane (PIN)
(not prismane)
P-24.0 IntroductionP-24.0 INTRODUCTION
P-24.1 Definitions
P-24.2 Spiro ring systems with only monocyclic ring components
P-24.3 Monospiro ring systems containing two identical polycyclic ring components
P-24.4 Three identical polycyclic ring components spirofused together
P-24.5 Monospiro ring systems with different ring components at least one of which is a polycyclic ring system
P-24.6 Unbranched polyspiro ring systems with different ring components, one being a polycyclic ring system
P-24.7 Branched polyspiro ring systems
P-24.8 Spiro ring systems containing atoms with nonstandard bonding numbers
This Section is based on the recent publication ‘Extension and Revision of the Nomenclature for Spiro Compounds, IUPAC Recommendations1999’ (ref. 8). It supersedes Rules A-41, A-43, B-10, and B-12 in the 1979 Recommendations (ref. 1) and Rule R-2.4.3 in the 1993 Recommendations (ref. 2). The alternative methods given by Rules A-42 and B-11 in the 1979 recommendations (ref. 1) have been abandoned.
Section P-24.5.2 takes into account appropriate modifications to Section SP-4.1 of the 1999 publication (ref. 8). Also in P-24.5.3, P-24.6, P-24.7 and P-24.8.4 some modifications on the usage of brackets have been made to SP-4, SP-5 and SP-7 of the 1999 publication (ref. 8).
The spiro ring systems consisting only of monocyclic rings in this Section are saturated systems; for unsaturated systems, see Section P-31.1.5.1.
For naming substituent groups see Sections P-29 and P-32.1.3.
P-24.1 DEFINITIONS
A ‘spiro union’ is a linkage between two rings that consists of a single atom common to both rings. A ‘free spiro union’ is a linkage that constitutes the only direct union between the two rings.
a non-free spiro union
Spirofusion is the creation of one, and only one, common atom between two rings or ring systems, each ring or ring system contributing one, and only one, atom to the spiro ring system. It is analogous to the ortho- or ortho- and peri-fusion that creates common bonds between mancude rings or ring systems. Traditionally, ortho- or ortho- and peri-fusion has been called ‘fusion’, with no reference to its specific type of fusion. To avoid any ambiguity, the term ‘spiro’ must always be specified when added to the term ‘fusion’.
P-24.2 SPIRO RING SYSTEMS WITH ONLY MONOCYCLIC RING COMPONENTS
P-24.2.0 IntroductionP-24.2.0 Introduction
P-24.2.1 Monospiro alicyclic ring systems
P-24.2.2 Linear polyspiro alicyclic ring systems
P-24.2.3 Branched polyspiro alicyclic ring systems
P-24.2.4 Heterocyclic spiro ring systems
This Section is concerned only with saturated spiro ring systems that consist only of monocyclic rings. For unsaturated alicyclic spiro ring systems, see Section P-31.1.5.
P-24.2.1 Monospiro alicyclic ring systems
Monospiro parent hydrides consisting of two saturated cycloalkane rings are named by placing the nondetachable prefix ‘spiro’ before the name of the unbranched acyclic hydrocarbon with the same total number of skeletal atoms. The number of skeletal atoms linked to the spiro atom in each ring is indicated by arabic numbers separated by a full stop, cited in ascending order and enclosed in square brackets; this descriptor (called in these recommendations the ‘von Baeyer spiro descriptor’) is placed between the spiro prefix and the name of the acyclic alkane. Numbering starts in the smaller ring, if one is smaller, at a ring atom next to the spiro atom and proceeds first around that ring, then through the spiro atom and around the second ring.
Examples:
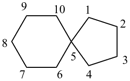
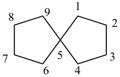
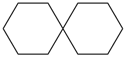
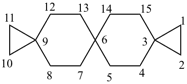
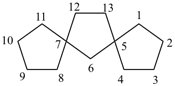
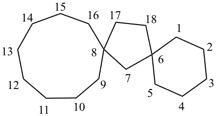
spiro[4.4]nonane (PIN)
Polyspiro parent hydrides consisting of unbranched assemblies of three or more saturated cycloalkane rings are named using the nondetachable prefixes ‘dispiro’, ‘trispiro’, etc., according to the number of spiro atoms present, cited in front of the name of the acyclic hydrocarbon that has the same number of skeletal atoms. The von Baeyer spiro descriptor indicates the number of carbon atoms linking the spiro atoms by arabic numbers that are cited in order, starting at the smaller terminal ring if one is smaller, and proceeding consecutively, always by the shorter path, to the other terminal ring through each spiro atom and then back to the first spiro atom; the numbers are separated by full stops and enclosed in square brackets. The compound is numbered in the order in which the numbers of the von Baeyer spiro descriptor are cited, including spiro atoms when encountered for the first time. Each time a spiro atom is reached for the second time its locant, which has already been assigned, is cited as a superscript number to the number of the preceding linking atoms.
Examples:
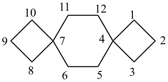
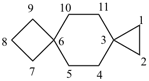
dispiro[2.2.36.23]undecane (PIN)
trispiro[2.2.2.29.26.23]pentadecane (PIN)
P-24.2.2.1 If there is a choice of numbers for the spiro descriptor, the smaller numbers are selected because low locants must be allocated to spiro atoms.
Examples:
 | not | 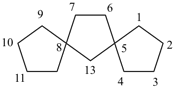 |
| dispiro[4.1.47.25]tridecane (PIN) correct | dispiro[4.2.48.15]tridecane incorrect |
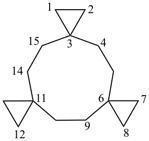
 | not | 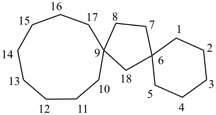 |
| dispiro[5.1.88.26]octadecane (PIN) correct | dispiro[5.2.89.16]octadecane incorrect |
P-24.2.2.2 If there is still a choice of numbering, the numbers of the von Baeyer descriptors are considered in the sequence of the order of their citation. The name is selected with lower numbers at the first point of difference.
Example:
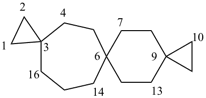 | not | 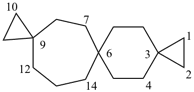 |
| trispiro[2.2.2.29.26.33]hexadecane (PIN) correct | trispiro[2.2.2.29.36.23]hexadecane incorrect |
P-24.2.3 Branched polyspiro alicyclic ring systems
Branched polyspiro hydrocarbons composed only of cycloalkane rings are named using ‘dispiro’, ‘trispiro’, etc. before the name of the acyclic hydrocarbon corresponding to the total number of skeletal atoms present. The von Baeyer spiro descriptor indicates the number of skeletal atoms linking the spiro atoms by arabic numbers that are cited in order, starting at the smallest terminal ring, if one is smallest, proceeding consecutively to succeeding terminal rings through each spiro atom, always by the shortest path, and then back to the first spiro atom; the numbers are separated by full stops and enclosed in square brackets. The compound is numbered in the order in which the numbers of the spiro von Baeyer descriptor are cited, including spiro atoms when encountered for the first time. Each time a spiro atom is reached for the second time its locant is cited as a superscript number to the preceding number of linking atoms.
Examples:
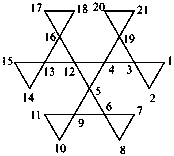
nonaspiro[2.0.0.0.26.0.29.05.0.0.213.0.216.012.04.0.219.03]henicosane (PIN)
Examples:
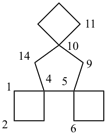 | not | 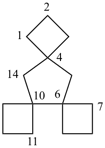 |
| trispiro[3.0.35.1.310.14]tetradecane (PIN) correct | trispiro[3.1.36.0.310.14]tetradecane incorrect |
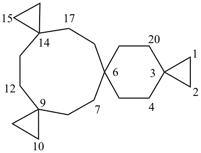 | not | 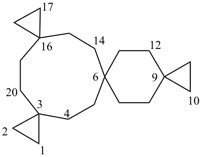 | not | 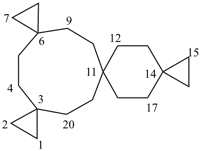 |
| tetraspiro[2.2.2.29.2.214.26.23]icosane (PIN) correct | tetraspiro[2.2.2.29.26.2.216.23]icosane incorrect | tetraspiro[2.2.26.2.2.214.211.23]icosane incorrect |
P-24.2.3.2 If there is still a choice of numbering, the numbers of the von Baeyer descriptor are considered in their order of citation. The correct name has the lower numbers at first point of difference.
Example:
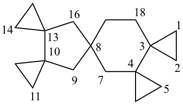 | not | 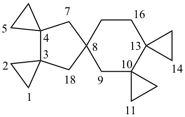 |
| pentaspiro[2.0.24.1.1.210.0.213.18.23]octadecane (PIN) correct | pentaspiro[2.0.24.1.1.210.0.213.28.13]octadecane incorrect |
P-24.2.4 Heterocyclic spiro ring systems
P-24.2.4.1 Heterocyclic spiro ring systems named by skeletal replacement (‘a’) nomenclatureP-24.2.4.1 Heterocyclic spiro ring systems named by skeletal replacement (‘a’) nomenclature
P-24.2.4.2 Homogeneous heterocyclic spiro ring systems with only monocyclic ring components
P-24.2.4.3 Heterocyclic spiro ring systems with only monocyclic ring components composed of alternating heteroatoms
P-24.2.4.1.1 When heteroatoms are present in a spiro ring system composed of only monocyclic rings, skeletal replacement (‘a’) nomenclature is used to name the heterocyclic system. The name of the corresponding hydrocarbon ring system is first constructed as described above (P-24.2.1, P-24.2.2, P-24.2.3). Heteroatoms are then introduced by using the general principles of skeletal replacement (‘a’) nomenclature. The numbering of the spiro hydrocarbon ring system is never modified by the introduction of heteroatoms, but low locants must be attributed to heteroatoms if there is a choice.
Examples:
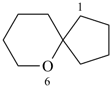
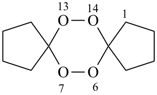
6,7,13,14-tetraoxadispiro[4.2.48.25]tetradecane (PIN)
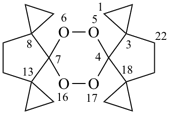
5,6,16,17-tetraoxahexaspiro[2.0.2.0.28.2.213.07.24.0.218.23]docosane (PIN)
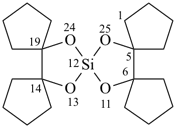
11,13,24,25-tetraoxa-12-silapentaspiro[4.0.46.1.1.414.0.419.112.15]pentacosane (PIN)
(a) low locants are allocated to heteroatoms as a set regardless of the kind of heteroatom;P-24.2.4.2 Homogeneous heterocyclic spiro ring systems with only monocyclic ring components
(b) if there is still a choice, low locants are assigned in accord with the following decreasing seniority order of heteroatoms: F > Cl > Br > I > O > S > Se > Te > N > P > As > Sb > Bi > Si > Ge > Sn > Pb > B > Al > Ga > In > Tl.
9-oxa-6-azaspiro[4.5]decane (PIN)Example:
7-thia-9-azaspiro[4.5]decane (PIN)
Heterocyclic spiro ring systems with only monocyclic ring components and composed entirely of the same heteroatom are named as described above using the name of the homogeneous heteroacyclic ring system that has the same total number of skeletal heteroatoms. This method is preferred to skeletal replacement (‘a’) nomenclature described in P-24.2.4.1, in which the total number of heteroatoms is indicated by a numerical term. In either method it is not necessary to give the location of the heteroatoms because all positions are modified by the same heteroatom. For the preselected names (see P-12.2) that are used to generate PIN names for organic derivatives see P-52.1.6.
Example:
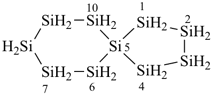
Heterocyclic spiro ring systems consisting only of monocyclic rings and having alternating skeletal heteroatoms are named by two methods.
(1) by citing a prefix such as ‘spiro’, ‘dispiro’, etc., before a von Baeyer descriptor (indicating the numbers of heteroatoms linked to each spiro atom in each ring cited in increasing order and separated by a full stop) enclosed in square brackets followed successively by:For the preselected names (see P-12.2) that are used to generate PIN names for organic derivatives see P-52.1.6.2.
(a) a multiplying prefix (‘di’, ‘tri’, etc) denoting the number of heteroatoms of the first cited ‘a’ term that follows;(2) by skeletal replacement (‘a’) nomenclature as described in P-24.2.4.1.(b) the ‘a’ terms of the hetero atoms, cited in the reverse seniority order for ‘a’ prefixes (see P-21.2.3.1), for example, Si before O;
(c) the ending ‘ane’. The hetero spiro system is numbered as is the corresponding hydrocarbon.
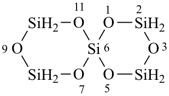
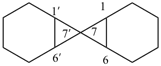
P-24.3.1 Monospiro ring systems consisting of two identical polycyclic ring components are named by placing the nondetachable prefix ‘spirobi’ before the name of the component ring system enclosed in square brackets. The established numbering system of the polycyclic ring system component is retained with one system having primed locants. The location of the spiro atom is indicated in the name by the appropriate locants (unprimed first) placed at the front of the name.
Example:
Note: This treatment of indicated hydrogen was introduced in the 1999 publication on the nomenclature of spiro compounds (ref. 8)
Examples:
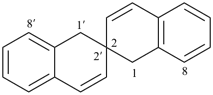
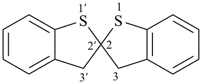
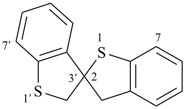
3H,3′H-2,2′-spirobi[[1]benzothiophene] (PIN)
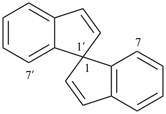
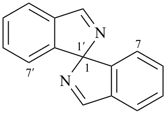
1,1′-spirobi[isoindole] (PIN)
Examples:
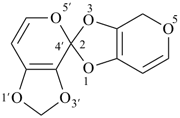
2′H,4H-2,4′-spirobi[[1,3]dioxolo[4,5-c]pyran] (PIN)
Note 2: The double set of brackets in this name occurs because the spiro name requires them and brackets are used to enclose locants belonging to component names (see P-16.5.2.2).
When ring components of ‘spirobi’ compounds are named by von Baeyer nomenclature, heteroatoms are indicated by skeletal replacement (‘a’) nomenclature. The spirobi ring system is named as the saturated bi- or polycyclic alicyclic hydrocarbon and the heteroatoms are denoted by ‘a’ prefixes cited at the front of the completed ‘spirobi’ hydrocarbon name. If there is a choice, low locants are given to the spiro atom, then to the heteroatoms as described in Section P-24.2.4.1.
Note: This treatment of skeletal replacement (‘a’) prefixes was introduced in the 1999 publication on the nomenclature of spiro compounds (ref. 8).
Examples:
 | not | 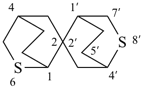 |
| (I) | (II) | |
| correct | incorrect | |
| 5,6′-dithia-2,2′-spirobi[bicyclo[2.2.2]octane] (I) (PIN) [not 6,8′-dithia-2,2′-spirobi[bicyclo[2.2.2]octane] (II); the set of locants ‘5,6′ ’ in (I) is lower than ‘6,8′ ’ in (II)] | ||
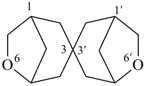
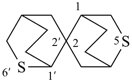
6,6′-dioxa-3,3′-spirobi[bicyclo[3.2.1]octane] (PIN)
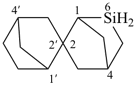
6-sila-2,2′-spirobi[bicyclo[2.2.1]heptane] (PIN)
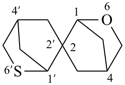
6-oxa-6′-thia-2,2′-spirobi[bicyclo[2.2.1]heptane] (PIN)
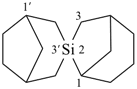
2-sila-2,3′-spirobi[bicyclo[3.2.1]octane] (PIN)
P-24.4.1 Dispiro ring systems with three identical polycyclic ring components are named by placing the nondetachable prefix ‘dispiroter’ before the name of the component ring system enclosed in square brackets. The multiplicative prefix ‘ter’ (see P-14.2.3) is used to indicate the repetition of identical ring components. Locants for the middle ring component are primed and for the third ring component double primed. The spiro atoms are indicated in front of the name by two pairs of locants separated by a colon. Indicated hydrogen is cited in front of these locants, if needed.
Examples:
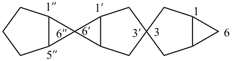
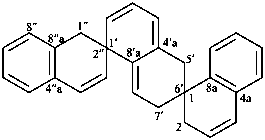
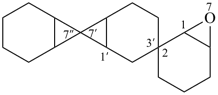
1H,1′H,1′′H,3′H-2,2′:7′,2′′-dispiroter[naphthalene] (PIN)
Examples:
 | not | 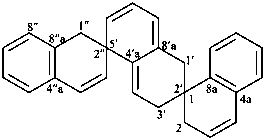 | nor | 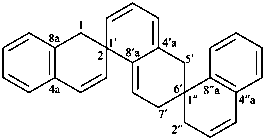 |
| (I) | (II) | (III) | ||
| correct | incorrect | incorrect | ||
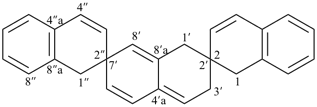
| 1′′H,2H,5′H,7′H-1,6′:1′,2′′-dispiroter[naphthalene] (I) (PIN) [not 1′H,1′′H,2H,3′H-1,2′:5′,2′′-dispiroter[naphthalene] (II); nor 1H,2′′H,5′H,7′H-2,1′:6′,1′′-dispiroter[naphthalene] (III) | ||||
P-24.4.3 Three identical heterocyclic ring components spirofused together.
Dispiro compounds with three identical heterocyclic components may be named:
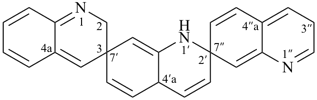
(a) by using heterocyclic monocyclic or polycyclic mancude components in the same way as for ‘spiroter’ hydrocarbons (see P-24.4.1); the numbering depends first on the fixed numbering of the heterocyclic components;Examples:(b) by skeletal replacement (‘a’) nomenclature when the ring components are polycyclic von Baeyer ring systems; the numbering of the ‘spiroter’ von Baeyer hydrocarbon remains unchanged.
 | not | 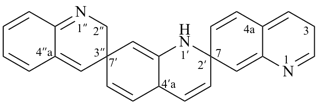 |
| correct | incorrect | |
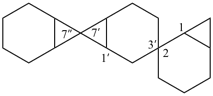
| 1′H,2H,3′′H,4′aH-3,7′:2′,7′′-dispiroter[quinoline] (PIN) | 1′H,2′′H,3H,4′aH-7,2′:7′,3′′-dispiroter[quinoline] |
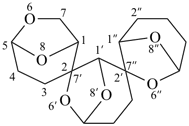
6,6′,6′′,8,8′,8′′-hexaoxa-2,7′:2′,7′′-dispiroter[bicyclo[3.2.1]octane] (PIN)
P-24.5.1 Monospiro ring systems with different ring components, at least one being a polycyclic ring system to which skeletal replacement (‘a’) nomenclature does not apply, are formed by placing the ring component names in alphanumerical order within square brackets. The position of the spiro atom is denoted by appropriate locants separated by a comma and placed between the names of the two ring components. Locants of the second ring component are primed and thus any locants needed to name it are placed in square brackets. Indicated hydrogen (see P-14.7) is cited in front of the name if needed in the complete structure (P-24.3.2).
Note: In naming polycyclic spiro ring systems consisting of different ring components, the first ring to be cited is determined by alphabetical order and not by seniority of the rings or ring systems.
Examples:
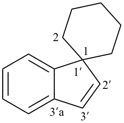
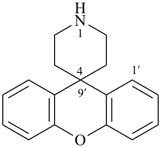
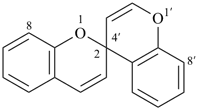
spiro[piperidine-4,9′-xanthene] (PIN)
(no indicated hydrogen is required, see P-24.3.2)
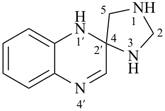
1′H-spiro[imidazolidine-4,2′-quinoxaline] (PIN)
Note: This treatment of skeletal replacement (‘a’) prefixes was introduced in the 1999 publication on the nomenclature of spiro compounds (ref. 8).
Examples:
 | not | 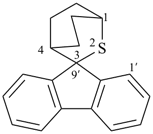 |
| (I) | (II) | |
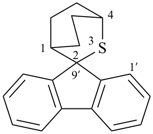
| correct | incorrect | |
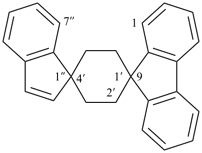
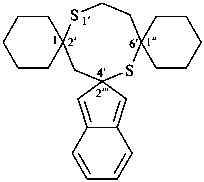
| 3-thiaspiro[bicyclo[2.2.2]octane-2,9′-fluorene] (I) (PIN) [not 2-thiaspiro[bicyclo[2.2.2]octane-3,9′-fluorene] (II)] | ||
Note: The format of the ‘correct’ name given in SP-4.1 in ref. 8, spiro[fluorene-9,2′-[3]thiabicyclo[2.2.2]octane], is no longer recommended.
 | not | 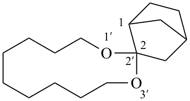 |
| (I) | (II) | |
| correct | incorrect | |
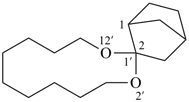
| 2′,12′-dioxaspiro[bicyclo[2.2.1]heptane-2,1′-cyclododecane] (I) (PIN) [not 1′,3′-dioxaspiro[bicyclo[2.2.1]heptane-2,2′-cyclododecane] (II)] | ||
Note: The format of the ‘correct’ name given in SP-4.1, ref. 8, spiro[bicyclo[2.2.1]heptane-2,1′-[2,12]dioxacyclododecane], is no longer recommended.
P-24.5.3 Alphanumerical order, as described in Section P-14.5 and P-14.6, is used when necessary. When Roman letters are inadequate to distinguish alphabetically between two ring components, criteria based on italic fusion letters and numbers, heteroatom locants, and von Baeyer descriptor numbers are used, as appropriate. All locants present in bicyclic fused benzo ring component or Hantzsch-Widman named component are placed in brackets (without primes for the second component, see third and fourth examples below).
Examples:
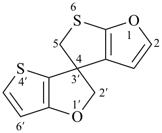
2′H,5H-spiro[thieno[2,3-b]furan-4,3′-thieno[3,2-b]furan] (PIN)
( ...[2,3-b]... before ...[3,2-b]...)
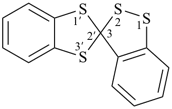
spiro[[1,2]benzodithiole-3,2′-[1,3]benzodithiole] (PIN)
(1,2-benzo... before 1,3-benzo...)
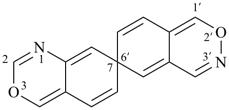
P-24.5.4 In the case of ring systems modified by skeletal replacement (‘a’) nomenclature, P-24.5.1 and P-24.5.3 are applied to name the ring system before skeletal replacement (‘a’) nomenclature is applied as described in P-24.5.2. The names of ring components modified by skeletal replacement (‘a’) nomenclature must not be used; thus P-24.5.3 must be applied when required.
Examples:
 | not | 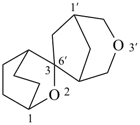 |
| (I) | (II) | |
| correct | incorrect | |
| 3,3′-dioxaspiro[bicyclo[2.2.2]octane-2,6′-bicyclo[3.2.1]octane] (I) (PIN) [not 2,3′-dioxaspiro[bicyclo[2.2.2]octane-3,6′-bicyclo[3.2.1]octane] (II)] | ||
P-24.6 UNBRANCHED POLYSPIRO RING SYSTEMS WITH DIFFERENT RING COMPONENTS ONE BEING A POLYCYCLIC RING SYSTEM
Unbranched polyspiro ring systems with at least two different ring components and at least one of which is a polycyclic ring are named by placing the ring component names in order of their occurrence in the structure beginning with the terminal ring component lower in alphabetical order and enclosing within square brackets. A nondetachable prefix indicating the number of spiro atoms (‘dispiro’, ‘trispiro’, etc.) is placed in front of the enclosed ring component names. Locants of the first cited ring component are unprimed, the next ring component is primed, and so on. In a complete name, all locants present in a bicyclic fused benzo ring component or Hantzsch-Widman named component are placed in brackets (without primes for the second and subsequent components, see fourth example below). The positions of the spiro atoms are indicated by the appropriate pair of locants separated by a comma and placed between each pair of component ring system names. Indicated hydrogen is used as needed and cited in front of the ‘dispiro’, ‘trispiro’, etc., prefix. If both terminal ring systems are the same, the order for citation of ring system components is determined by comparing the pair of second ring components from the end of the structure, and so on (See section SP-5 in ref. 8 for further discussion).
Examples:
2′′H,4′′H-trispiro[cyclohexane-1,1′-cyclopentane-3′,3′′-cyclopenta[b]pyran-6′′,1′′′-cyclohexane] (PIN)
7′-azadispiro[fluorene-9,2′-bicyclo[2.2.1]heptane-5′,1′′-indene] (PIN)
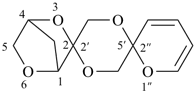
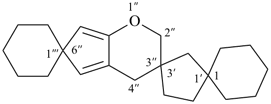
3,6-dioxadispiro[bicyclo[2.2.1]heptane-2,2′-[1,4]dioxane-5′,2′′-pyran] (PIN)
Example:
 | not |  | not |  |
| (I) | (II) | (III) | ||
| correct | incorrect | incorrect | ||
| dispiro[[1,3]dioxolane-2,3′-bicyclo[3.2.1]octane-6′,2′′-[1,3]dioxolane] (I) (PIN) [not dispiro[[1,3]dioxolane-2,6′-bicyclo[3.2.1]octane-3′,2′′-[1,3]dioxolane] (II); the locant sets 2,2′′,3′,6′ are identical, but in the name the order of citation 2,3′,6′,2′′ in the PIN (I) is lower than 2,6′,3′,2′′ in (II)] [not dispiro[bicyclo[3.2.1]octane-3,2′:6,2′′-bis([1,3]dioxolane) (III) (see P-24.4.2)] | ||||
P-24.7 BRANCHED POLYSPIRO RING SYSTEMS
When three or more components are spirofused to another single component, the system is described as a branched spirofused system. Terminal components have only one spiro atom.
P-24.7.1 When a central ring component is spirofused to three or more identical terminal ring components, the central ring component is cited first and its locants are unprimed. The terminal ring components are cited with the appropriate multiplicative prefix (‘tris’, ‘tetrakis’, etc.) and their locants are primed, double primed, etc., in accordance with the lowest possible locants of the spiro atoms of the central ring component. The spiro atoms are indicated by pairs of locants separated by a colon. Indicated hydrogen is cited as necessary in front of the appropriate spiro prefix.
Example:
Examples:
| (1) | 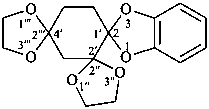 |
| trispiro[[1,3]benzodioxole-2,1′-cyclohexane-2′,2′′:4′,2′′′-bis([1,3]dioxolane)] (PIN) |
| (2) | 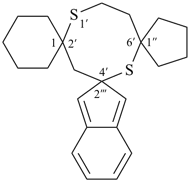 |
| trispiro[cyclohexane-1,2′-[1,5]dithiocane-6′,1′′-cyclopentane-4′,2′′′-indene] (PIN) (the cyclohexane ring must be spirofused at position ‘2’ of the dithiocane central ring component) |
| (3) | 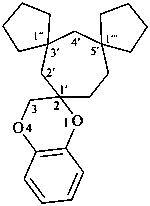 | not | 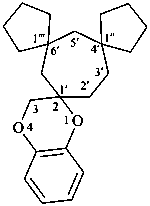 |
| (I) | (II) | ||
| correct | incorrect | ||
| 3H-trispiro[[1,4]benzodioxine-2,1′-cycloheptane-3′,1′′:5′,1′′′-bis(cyclopentane)] (I) (PIN) {not 3H-trispiro[[1,4]benzodioxine-2,1′-cycloheptane-4′,1′′:6′,1′′′-bis(cyclopentane)] (II)} | |||
| (4) | 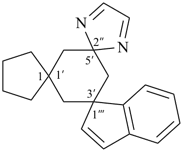 | not | 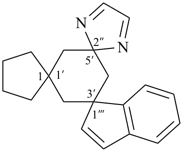 |
| (I) | (II) | ||
| correct | incorrect | ||
| trispiro[cyclopentane-1,1′-cyclohexane-3′,2′′-imidazole-5′,1′′′-indene] (I) (PIN) [not trispiro[cyclopentane-1,1′-cyclohexane-5′,2′′-imidazole-3′,1′′′-indene] (II) | |||
P-24.7.3 If no decision can be attained by application of P-24.7.2 and if there is a choice of locants, the lowest set of locants for all spiro atoms is selected when compared as a set in increasing numerical order, and, if still undecided, in their order of their citation in the name. If a choice still remains, criteria about heteroatoms and indicated hydrogen are taken into consideration (see Section SP-3.2 in ref. 8).
Example:
 | not | 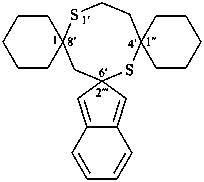 | |
| (I) | (II) | ||
| correct | incorrect | ||
| nor | 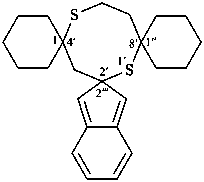 | nor | 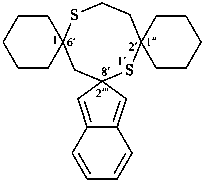 |
| (III) | (IV) | ||
| incorrect | incorrect | ||
| trispiro[bis(cyclohexane)-1,2′:6′,1′′-[1,5]dithiocane-4′,2′′′-indene] (I) (PIN) [not trispiro[bis(cyclohexane)-1,8′:4′,1′′-[1,5]dithiocane-6′,2′′′-indene] (II) nor trispiro[bis(cyclohexane)-1,4′:8′,1′′-[1,5]dithiocane-2′,2′′′-indene] (III); nor trispiro[bis(cyclohexane)-1,6′:2′,1′′-[1,5]dithiocane-8′,2′′′-indene] (IV)] | |||
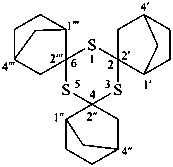
P-24.7.4 If additional components are spiro-fused to a branched polyspiro compound as described in P-24.7.1 to P-24.7.3, the following criteria are applied in order:
(a) Any monocyclic ring components spirofused together including skeletal replacement (‘a’) prefixes, if any, are named (P-24.2) as a unit containing the maximum number of monocyclic ring components. The unit is used as a component for further spiro-fusion (see P-24.6). To help indicate the composite nature of the name braces are used (instead of brackets) after the initial polyspiro prefix to enclose components at least one of which is already spiro-fused;P-24.7.4.1 After identification of the spiro-fused components these, together with the remaining components, are treated in the normal way.(b) If there is not a polyspiro system of monocyclic ring components or the system cannot be named by the normal spiro-fusion described previously the largest spiro system is named and treated as a unit for further spiro-fusion. The priming of the unit is continued to the rest of the name.
Examples:
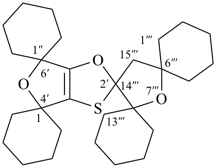
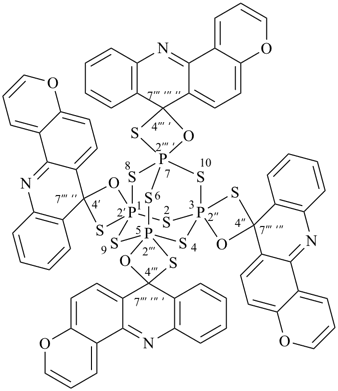
1λ5,3λ5,5λ5,7λ5-tetraspiro{tetraspiro[2,4,6,8,9,10-hexathia-1,3,5,7-tetraphosphaadamantane-1,2′:3,2′′:5,2′′′:7,2′′′ ′-
tetrakis([1,3,2]oxathiaphosphetane)-4′,7′′′ ′′:4′′,7′′′ ′′′:4′′′,7′′′ ′′′ ′:4′′′ ′,7′′′ ′′′ ′′-tetrakis(pyrano[2,3-c]acridine)} [PIN, see SP-6.4 (b) in ref 8]
octaspiro[2,4,6,8,9,10-hexathia-1,3,5,7-tetraphosphatricyclo[3.3.1.13,7]decane-1,2′λ5:3,2′′λ5:5,2′′′λ5:7,2′′′′λ5-
tetrakis[1,3,2]oxathiaphosphetane-4′,7′′′′′:4′′,7′′′′′′:4′′′,7′′′′′′′:4′′′′,7′′′′′′′′-tetrakis[7H]pyrano[2,3-c]acridine]
(the CAS index name; note that multiple primes are not divided into groups of three)
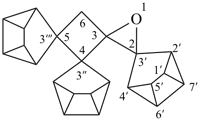
trispiro{1-oxaspiro[2.3]hexane-2,3′:4,3′′:5,3′′′-tris(tetracyclo[3.2.0.02,7.04,6]heptane)} (PIN)
The λ-convention, characterized by the symbol λn, is used to identify heteroatoms with nonstandard bonding numbers (see P-14.1). The symbol is placed at the front of the complete name or before the skeletal replacement (‘a’) prefix for the atom to which it refers.
P-24.8.1 Spiro ring systems with only monocyclic ring componentsP-24.8.1 Spiro ring systems with only monocyclic ring components
P-24.8.2 Monospiro ring systems with two identical polycyclic ring components
P-24.8.3 Spiro ring systems with three identical components and one nonstandard spiro atom
P-24.8.4 Monospiro ring systems with different ring components, at least one of which is a polycyclic ring with a nonstandard spiro atom
P-24.8.5 Unbranched polyspiro ring systems with different ring components, at least one of which is a polycyclic ring with at least one nonstandard spiro atom
P-24.8.6 Branched spiro ring systems with at least one polycyclic ring component
P-24.8.1.1 Heteroatoms having nonstandard bonding numbers receive lowest locants in accordance with the numbering of the corresponding spiro ring system.
Examples:
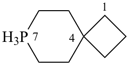
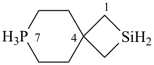
7λ5-phospha-2-silaspiro[3.5]nonane (PIN)
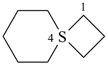
4λ4-thiaspiro[3.5]nonane (PIN)
Example:
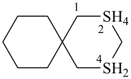
P-24.8.1.3.1 Ring systems consisting of three monocyclic rings and one nonstandard spiro atom (e.g., a λ6 spiro atom) are named by placing the prefix ‘spiro’ before the name corresponding to an alicyclic system with the same total number of skeletal atoms in the spiro ring system. Heteroatoms are indicated by ‘a’ prefixes and the nonstandard bonding number by the ‘λ’ symbol (see P-14.1). In the von Baeyer spiro descriptor, the locant of the spiro atom is used as a superscript number to indicate each time the spiro atom is revisited.
Example:
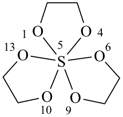
Example:
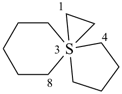
(a) Low numbers are selected for spiro atoms;
Example:
 | not | 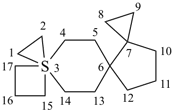 | not | 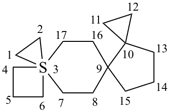 |
| (I) | (II) | (III) | ||
| correct | incorrect | incorrect | ||
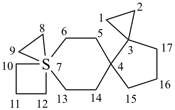
| 7λ6-thiatrispiro[2.0.2.27.37.24.33]heptadecane (I) (PIN) [not 3λ6-thiatrispiro[2.2.0.27.36.23.33]heptadecane (II);nor 3λ6-thiatrispiro[2.3.2.0.210.39.23]heptadecane (III)] | ||||
(b) Low numbers are selected for spiro atoms connecting three rings;
Example:
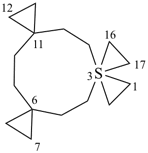 | not | 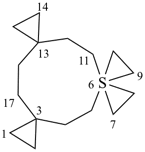 | not | 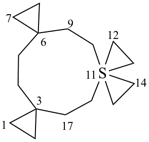 |
| (I) | (II) | (III) | ||
| correct | incorrect | incorrect | ||
| 3λ6-thiatrispiro[2.2.26.2.211.23.23]heptadecane (I) (PIN) [not 6λ6-thiatrispiro[2.2.26.26.2.213.23]heptadecane (II);nor 11λ6-thiatrispiro[2.2.26.2.211.211.23]heptadecane (III)] | ||||
(c) Low numbers are selected for the von Baeyer spiro descriptor in order of citation;
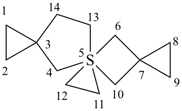
Example:
 | not | 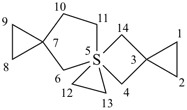 |
| (I) | (II) | |
| correct | incorrect | |
| 5λ6-thiatrispiro[2.1.1.27.15.25.23]tetradecane (I) (PIN) [not 5λ6-thiatrispiro[2.1.1.27.25.25.13]tetradecane (II)] | ||
P-24.8.2 Monospiro ring systems with two identical polycyclic ring components
The λn symbol is placed at the front of the complete name formed from the names of two identical ring system components including the heteroatoms in their name; the λn symbol is preceded by the lowest locant denoting the spiro atom. If indicated hydrogen atoms are required, they are placed before the λ symbol. When there is a choice, the locant of the first cited component is used for indicated hydrogen.
Note: The ‘lowest locant’ is used as the criterion for identification of the λn spiro atom rather than ‘least primed locant‘ as used in SP-7 in ref. 8.
Examples:
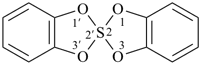
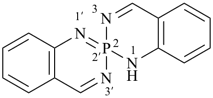
P-24.8.3 Spiro ring systems with three identical components and one nonstandard spiro atom.
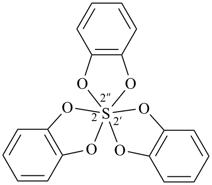
Ring systems composed of three identical polycyclic ring components and only one spiro atom are named by placing the prefix ‘spiroter’ before the name of the polycyclic component enclosed in square brackets. The three spiro locants are cited at the front of the name preceded by the λ symbol with its locant that must be the lowest of the three denoting the spiro atom.
Example:
P-24.8.4 Monospiro ring systems with different ring components at least one of which is a polycyclic ring with a nonstandard spiro atom
P-24.8.4.1 Monospiro ring systems composed of two different ring systems and a spiro heteroatom with a nonstandard bonding number are named by placing the prefix spiro in front of the names of the components cited in alphabetical order and with appropriate spiro locants. The lowest locant (unprimed) is used to denote the spiro fusion and the λ symbol is placed at the front of the name. Indicated hydrogen, if necessary, is added in front of the λ symbol. Any additional atom with a nonstandard bonding number is treated as a part of the name of the heterocycle; the λ symbol is cited with the lowest locant denoting the spiro atom.
Examples:
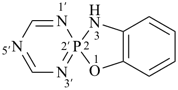
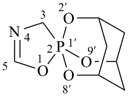
Note 2: The double set of brackets in this name occurs because the spiro name requires them and brackets are used to enclose locants belonging to ring component names (see P-16.5.2.2). The use of brackets in the name of the first cited component is a change from SP-4 of ref. 8.
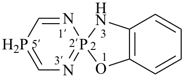
P-24.8.4.2 When three ring components represented by two different individual rings, are present, the name of the ring component that is cited second is placed in parentheses to highlight this unusual situation. The λn symbol is placed at the front of the name, preceded by the lowest locant denoting the spiro atom.
Example:
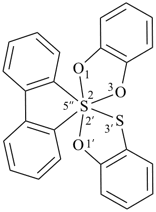
P-24.8.4.3 Two identical ring components are denoted by the prefix ‘bis’. The λn symbol is placed at the front of the name, preceded by the lowest locant denoting the spiro atom.
Example:
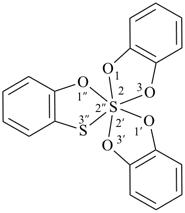
P-24.8.5 Unbranched polyspiro ring systems with different ring components at least one of which is a polycyclic ring with at least one nonstandard spiro atom
Unbranched polyspiro ring systems with different ring components at least one of which is a polycyclic ring system with at least one nonstandard spiro atom are named using the method described in P-24.6. The λ-symbol denoting a spiro junction is associated with the lowest locant and placed in front of the name; it is preceded by indicated hydrogen(s), as needed.
Example:
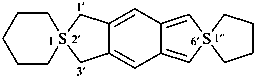
If two or more different terminal ring components are spirofused to a central ring component, the alphabetically earliest is cited first with multiplicative prefixes, if appropriate, followed by the central ring component and the remaining terminal ring components in alphabetical order. The λ symbol is placed at the front of the complete name and is denoted by the lowest spiro locant; it is preceded by indicated hydrogen, if needed.
Example:
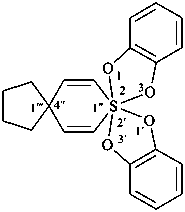
When there is a choice for locants, the lowest set of locants for all spiro atoms is selected, first by comparing them as a set in increasing numerical order, and, if still undecided, in the order of citation in the name. If a choice still remains, criteria involving the heteroatoms and indicated hydrogen atoms are taken into consideration (see Section SP-3.2 and SP-1.8 in ref. 8).
Example:
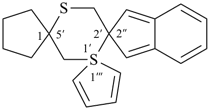 | not | 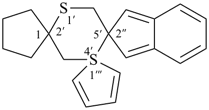 | not | 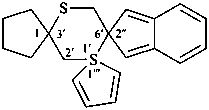 |
| (I) | (II) | (III) | ||
| correct | incorrect | incorrect | ||
| 1′λ4-trispiro[cyclopentane-1,5′-[1,4]dithiane-2′,2′′-indene-1′,1′′-thiophene] (I) (PIN) [not 4′λ4-trispiro[cyclopentane-1,2′-[1,4]dithiane-5′,2′′-indene-4′,1′′′-thiophene] (II)] nor 1′λ4-trispiro[cyclopentane-1,3′-[1,4]dithiane-6′,2′′-indene-1′,1′′′-thiophene] (III)] | ||||
P-25 FUSED AND BRIDGED FUSED RING SYSTEMS
P-25.0 IntroductionP-25.0 INTRODUCTION
P-25.1 Names of hydrocarbon parent ring components
P-25.2 Names of heterocyclic parent ring components
P-25.3 Constructing fusion names
P-25.4 Bridged fused ring systems
P-25.5 Limitations of fusion nomenclature: three components ortho- and peri-fused together
P-25.6 Fused ring systems with skeletal atoms with nonstandard bonding numbers
P-25.7 Double bonds, indicated hydrogen, and the δ-convention
P-25.8 Parent components in decreasing order of seniority (partial lists)
This section is based on the document entitled ‘Nomenclature of Fused and Bridged Fused Ring Systems, IUPAC Recommendations 1998’ (ref. 4).
In nomenclature, fusion is the operation that creates a common bond between two rings, each ring contributing one bond and the two atoms directly attached to the bond. This type of fusion is called ortho- or ortho- and peri-fusion if two adjacent bonds are involved. Atoms common to two or more rings are termed ‘fusion’ atoms. The term fusion is also used to describe the operation creating a common atom between two rings or ring systems, each contributing one atom. This type of fusion is called spirofusion (see P-24.1). Traditionally, ortho- and ortho- and peri-fusion were simply called fusion and the resulting polycyclic systems were referred to as fused ring systems or fused ring compounds. The term ‘spirofusion’ is new in nomenclature, and to avoid ambiguity ‘fusion’ should not be used without the prefix ‘spiro’ when ‘spirofusion’ is intended.
 | + |  |  | 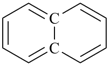 |
| benzene (PIN) | benzene (PIN) | naphthalene (PIN) |
 | + |  |  | 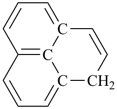 |
| naphthalene (PIN) | benzene (PIN) | 1H-phenalene (PIN) |
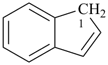 | + | 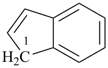 |  | 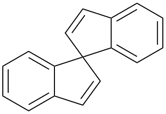 | |
| 1H-indene (PIN) | 1H-indene (PIN) | 1,1′-spirobi[indene] | (PIN) |
This section deals with fused (ortho- and ortho- and peri-fused) ring systems and bridged fused (ortho- and ortho- and peri-fused) ring systems. Spirofusion is described in Section P-24. This section is intended only as an introduction to the vast field of fusion nomenclature discussed in the document entitled ‘Nomenclature of Fused and Bridged Fused Ring Systems’ (ref. 4). The principles presented herein use rather simple examples; for more complex ring systems the publication noted above or the ‘Ring Systems Handbook’, published by the Chemical Abstracts Service (ref. 22), should be consulted. Changes from previous rules are highlighted.
P-25.1 NAMES OF HYDROCARBON PARENT RING COMPONENTS
P-25.1.1 Retained names for hydrocarbons used for parent ring components and as attached ring componentsP-25.1.1 Retained names for hydrocarbons used for parent ring components and as attached ring components
P-25.1.2 Systematically named hydrocarbon parent components
Retained names (also called trivial names) for polycyclic hydrocarbons are listed in Table 2.7, in decreasing order of seniority for being chosen as parent components in fusion nomenclature. Their numbering is indicated as the result of the application of the specific criteria used to number fused ring systems described in Section P-25.3.3.
(1) ovalene (PIN)
(2) pyranthrene (PIN)
(3) coronene (PIN)
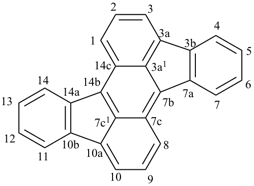
(4) rubicene (PIN)
(5) perylene (PIN)
(6) picene (PIN)
(7) pleiadene (PIN)
(8) chrysene (PIN)
(9) pyrene (PIN)
(10) fluoranthene (PIN)
(11) anthracene (PIN)
(special numbering)
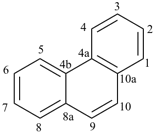
(12) phenanthrene (PIN)
(special numbering)
(13) phenalene
(1H-isomer shown; the PIN is 1H-phenalene)
(14) fluorene
(9H-isomer shown; the PIN is 9H-fluorene)
(15) s-indacene (PIN)
(16) as-indacene (PIN)
(17) azulene (PIN)
(18) naphthalene (PIN)
(19) indene
(1H-isomer shown; the PIN is 1H-indene)
Names for some hydrocarbon parent components having the maximum number of noncumulative double bonds (called mancude ring systems) and having at least two rings of five or more ring members are systematically formed using a prefix and an ending or term representing the nature and arrangement of the component rings. Rules for numbering are described in Section P-25.3.3.
P-25.1.2.1 PolyacenesP-25.1.2.1 Polyacenes. A hydrocarbon parent component consisting of four or more ortho-fused benzene rings in a straight linear arrangement is named by citing a numerical prefix (‘tetra’, ‘penta’, etc.) denoting the number of rings followed by the ending ‘acene’ (derived from the retained name anthracene) with elision of a letter ‘a’.
P-25.1.2.2 Polyaphenes
P-25.1.2.3 Polyalenes
P-25.1.2.4 Polyphenylenes
P-25.1.2.5 Polynaphthylenes
P-25.1.2.6 Polyhelicenes
P-25.1.2.7 Ace...ylenes
Examples:
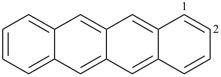
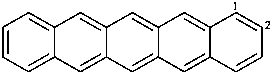
pentacene (PIN)
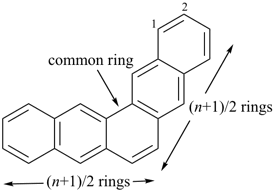
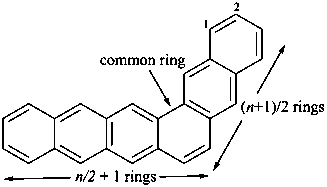
hexaphene (n = 6) (PIN)
Examples:
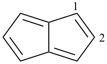
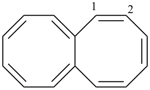
octalene (PIN)
Examples:
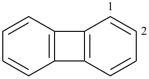
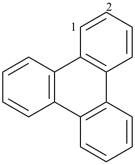
triphenylene (PIN)
Examples:
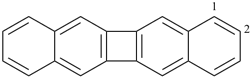
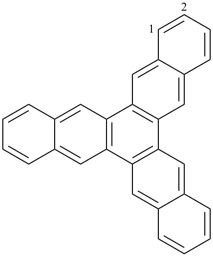
trinaphthylene (PIN)
Note: The definition, orientation, and numbering of polyhelicenes was changed in the comprehensive fused ring nomenclature document ‘Nomenclature of Fused and Bridged Fused Ring Systems’ (ref. 4). The series begins with six rings and not five rings as indicated in the 1993 Guide (R-2.4.1.3.6 in ref. 2) and in the Glossary of Class Names (ref. 23). The new orientation and numbering are presented in Section P-25.3.3.1.1.
Example:
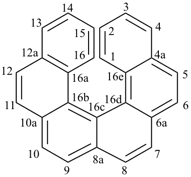 | 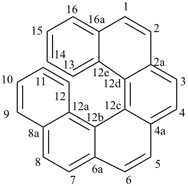 | |
| hexahelicene (PIN) (new orientation and numbering) | Note: Orientation and numbering no longer recommended; but still in use by CAS for the ring system having the name phenanthro[3,4-c]phenanthrene, ref. 22) |
Examples:
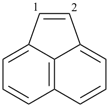
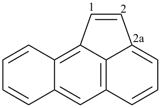
aceanthrylene (PIN)
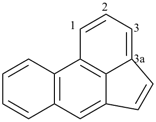
acephenanthrylene (PIN)
P-25.2.1 Retained names used for parent components and as attached componentsP-25.2.1 Retained names for heterocycles (also called trivial names) with the maximum number of noncumulative double bonds (called mancude ring systems) used for parent components and as attached components are given in Table 2.8.
P-25.2.2 Names formed systematically using endings and prefixes used for parent components and attached components
Ring systems are arranged in decreasing order of seniority for parent compounds in accordance with the seniority order described in Section P-25.3.2.4 and exemplified in Section P-25.8.1.
Skeletal replacement nomenclature, as described in Section P-15.5.2 , is used to replace O by S, Se, and Te of chromene, isochromene, and xanthene (PIN) to generate names for chalcogen analogues of these ring systems (see Table 2.8). Some names listed in Table 2.8 can be modified by a system of replacement specific to some nitrogen-containing compounds, in which N is replaced by As or P. The modified names are listed in Table 2.9; the modifiable compounds are marked by the symbol * in Table 2.8. Rules for numbering are described in Section P-25.3.3.
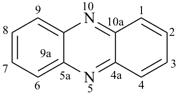
(1) phenazine (PIN)
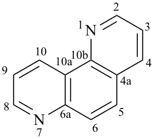
(2) phenanthroline
1,7-isomer shown; the PIN is 1,7-phenanthroline;
other isomers are: 1,8-; 1,9-; 1,10-; 2,7-; 2,8-; 2,9-; 3,7-; 3,8-; 4,7-)
(3) perimidine (1H-isomer shown; the PIN is 1H-perimidine)
(4) acridine* (PIN)
(special numbering)
(5) phenanthridine* (PIN)
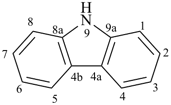
(6) carbazole
(special numbering; 9H-isomer shown; the PIN is 9H-carbazole)
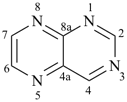
(7) pteridine (PIN)
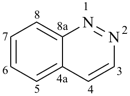
(8) cinnoline (PIN)
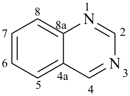
(9) quinazoline (PIN)
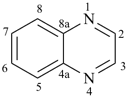
(10) quinoxaline (PIN)
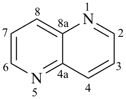
(11) naphthyridine
(1,5-isomer shown; the PIN is 1,5-naphthyridine;
other isomers are 1,6-; 1,7-; 1,8-; 2,6-; 2,7-)
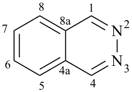
(12) phthalazine (PIN)
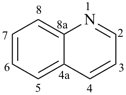
(13) quinoline*† (PIN)
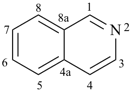
(14) isoquinoline*† (PIN)
(15) quinolizine*†
(4H-isomer shown; the PIN is 4H-quinolizine)
(16) purine
(special numbering, 7H-isomer shown; the PIN is 7H-purine)
(17) indazole
(1H-isomer shown; the PIN is 1H-indazole)
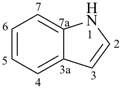
(18) indole*
(1H-isomer shown; the PIN is 1H-indole)
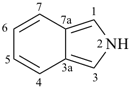
(19) isoindole*
(2H-isomer shown; the PIN is 2H-isoindole)
(20) indolizine* (PIN)
(21) pyrrolizine (1H-isomer shown; the PIN is 1H-pyrrolizine)
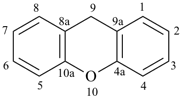
(22) xanthene
(special numbering; 9H-isomer shown; the PIN is 9H-xanthene)
thioxanthene (S instead of O)
(special numbering; 9H-isomer shown; the PIN is 9H-thioxanthene)
selenoxanthene (Se instead of O)
(special numbering; 9H-isomer shown; the PIN is 9H-selenoxanthene)
telluroxanthene (Te instead of O)
(special numbering; 9H-isomer shown; the PIN is 9H-telluroxanthene)
(23) chromene
(2H-isomer shown)
1-benzopyran
(2H-isomer shown; the PIN is 2H-1-benzopyran)
thiochromene (S instead of O)
(2H-isomer shown)
1-benzothiopyran (S instead of O)
(2H-isomer shown; the PIN is 2H-1-benzothiopyran
selenochromene (Se instead of O)
(2H-isomer shown)
1-benzoselenopyran (Se instead of O)
(2H-isomer shown; the PIN is 2H-1-benzoselenopyran)
tellurochromene (Te instead of O)
(2H-isomer shown)
1-benzotelluropyran (Te instead of O)
(2H-isomer shown; the PIN is 2H-1-benzotelluropyran)
(24) isochromene
(1H-isomer shown)
2-benzopyran
(1H-isomer shown; the PIN is 1H-2-benzopyran)
isothiochromene (S instead of O)
(1H-isomer shown)
2-benzothiopyran (S instead of O)
(1H-isomer shown; the PIN is 1H-2-benzothiopyran)
isoselenochromene (Se instead of O)
(1H-isomer shown)
2-benzoselenopyran (Se instead of O)
(1H-isomer shown; the PIN is 1H-2-benzoselenopyran)
isotellurochromene (Te instead of O)
(1H-isomer shown)
2-benzotelluropyran (Te instead of O)
(1H-isomer shown; the PIN is 1H-2-benzotelluropyran)
† In CAS index nomenclature, quinolizine precedes quinoline and isoquinoline in seniority.
Table 2.9 Names for nitrogenous parent components modified by phosphorus and arsenic replacement
(For the seniority of the phosphorus and arsenic ring systems see P-25.3.2.4 and P-25.8.1)
In the following names, arsenic or phosphorus atoms replace nitrogen atoms
| Nitrogen ring system | Arsenic ring system | Phosphorus ring system |
| acridine (PIN) | acridarsine (PIN)* | acridophosphine (PIN)* |
| indole (PIN) | arsindole (PIN) | phosphindole (PIN) |
| indolizine (PIN) | arsindolizine (PIN) | phosphindolizine (PIN) |
| isoindole (PIN) | isoarsindole (PIN) | isophosphindole (PIN) |
| isoquinoline (PIN) † | isoarsinoline (PIN) | isophosphinoline (PIN) |
| phenanthridine (PIN) | arsanthridine (PIN) | phosphanthridine (PIN) |
| quinoline (PIN) † | arsinoline (PIN) | phosphinoline (PIN) |
| quinolizine (PIN) † | rsinolizine (PIN) | phosphinolizine (PIN) |
* Numbered systematically, not as acridine.
† In CAS index nomenclature, quinolizine precedes quinoline and isoquinoline in seniority.
P-25.2.2.1 Heteromonocyclic parent componentsP-25.2.2.1 Heteromonocyclic parent components
P-25.2.2.2 Heteranthrene components
P-25.2.2.3 Pheno...ine components
P-25.2.2.4 Heteromonocyclic components fused to a benzene ring
P-25.2.2.1.1 Heteromonocyclic rings with three through ten ring members having the maximum number of noncumulative double bonds (called mancude ring systems) are used as parent components as well as attached components. Retained names are given in Table 2.2. Hantzsch-Widman names are discussed in P-22.2.2.
P-25.2.2.1.2 Names of heteromonocyclic parent components with more than ten ring members used in fusion nomenclature are discussed in this subsection; they are used only in fusion nomenclature (see also P-22.2.4). The preferred IUPAC names for such rings are ‘polyene’ names (see P-22.2.4).
A heteromonocyclic parent component having more than ten members and the maximum number of noncumulative double bonds (a mancude ring system) may be named by changing the ending ‘ane’ of the corresponding saturated heteromonocycle (see P-22.2.4) to ‘ine’. Their locants are cited in front of the name following indicated hydrogen, if any, in the order of the appearance of the corresponding replacement (‘a’) prefixes in the name.
For examples of fusion compounds including this type of heteromonocyclic parent component, see P-25.2.2.4.
Examples:
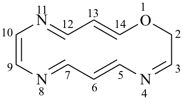
2H-1,4,8,11-oxatriazacyclotetradecine
1-oxa-4,8,11-triazacyclotetradeca-3,5,7,9,11,13-hexaene (PIN)
A heterotricyclic parent component consisting of two benzene rings fused to a 1,4-dihetera-benzene in which the heteroatoms are the same is named by attaching the appropriate ‘a’ prefix to the ending ‘anthrene’ (derived from anthracene), with elision of a letter ‘a’. The allowed heteroatoms are O, S, Se, Te, P, As, Si, and B. When the heteroatoms are nitrogen atoms, the component is named ‘phenazine’ (a retained name). The numbering is standard, as shown. Rules for numbering are described in Section P-25.3.3.
Examples:
X = S thianthrene (PIN)
X = Se selenanthrene (PIN)
X = Te telluranthrene (PIN)
X = N phenazine (PIN)
(a retained name)
X = P phosphanthrene (PIN)
X = As arsanthrene (PIN)
X = B boranthrene (PIN)
X = SiH silanthrene (PIN)
A heterotricyclic parent component consisting of two benzene rings fused to a 1,4-dihetera-benzene in which the heteroatoms are different is named by adding the prefix ‘pheno’ (derived from phenanthrene) to the appropriate Hantzsch-Widman name (see P- 22.2.2), eliding the ‘o’ before a following vowel. Numbering is standard and depends on the nature of the heteroatoms. Rules for numbering are described in Section P-25.3.3.
Examples:
X = S phenothiazine (10H-isomer shown; the PIN is 10H-phenothiazine)
X = Se phenoselenazine (10H-isomer shown; the PIN is 10H-phenoselenazine)
X = Te phenotellurazine (10H-isomer shown; the PIN is 10H-phenotellurazine)
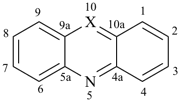
X = P phenazaphosphinine (PIN)
phenophosphazine (see refs. 2, 4)
X = As phenazarsinine (PIN)
phenarsazine (see refs. 2, 4)
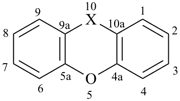
X = S phenoxathiine (PIN)
X = Se phenoxaselenine (PIN)
X = Te phenoxatellurine (PIN)
X = PH phenoxaphosphinine (PIN, 10H-isomer shown)
phenoxaphosphine (10H-isomer shown)
X = AsH phenoxarsinine (PIN, 10H-isomer shown)
phenoxarsine (10H-isomer shown)
X = SbH phenoxastibinine (PIN, 10H-isomer shown)
phenoxantimonine (10H-isomer shown)
X = AsH; and S instead of O phenothiarsinine (PIN, 10H-isomer shown)
phenothiarsine (10H-isomer shown)
If the initial identified preferred components for naming a fused ring system include an isolated benzo component (i.e. not forming part of a component with a retained name such as quinoline or anthracene) ortho-fused to a heteromonocyclic component of five or more members, these two components are treated together as a one-component unit (a benzoheterocycle). See P-25.3.5 for the use of benzoheterocycles in fusion nomenclature and limitations on its use. It is named by placing the locant(s) indicating the position(s) of the heteroatom(s) at the front of the name consisting of the fusion prefix ‘benzo’ followed by a retained name, a Hantzsch-Widman systematic name, or a name formed by skeletal replacement (‘a’) nomenclature as described in P-25.2.2.1.2. The locants cited correspond to the full bicyclic structure. As in Hantzsch-Widman names, locants are placed in the order corresponding to the order of citation of the heteroatoms in the heterocyclic component. The locant ‘1’ is always assigned to the atom of the heterocyclic component next to a fusion atom. Heteroatoms are allocated lowest locants as a set, without regard to kind; if there is a choice, lowest locants are assigned in accordance with the seniority of the ‘a’ prefixes (see Table 2.4). In general nomenclature locants may be omitted when the name is unambiguous; for preferred IUPAC names locants must be cited. The letter ‘o’ of the ‘benzo’ prefix is elided when followed by a vowel. Indicated hydrogen is placed at the front of the name, when required.
‘Benzo names’ offer several advantages. They are simpler in the sense that they do not require fusion descriptors. However, their primary advantage is in their use as components of fusion names; they provide a larger portion of structure and remove one full level of locants in the construction of names for larger heterocyclic fused ring systems.
Examples:
4H-3,1-benzoxazine (PIN)
4H-benzo[d][1,3]oxazine
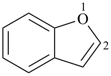
1-benzofuran (PIN)
benzofuran
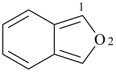
2-benzofuran (PIN)
isobenzofuran
benzo[c]furan
5,12-benzodioxacyclooctadecine (PIN)
benzo[m][1,8]dioxacyclooctadecine
1H-3-benzazacycloundecine (PIN)
1H-benzo[h][1]azacycloundecine
1H-3-benzoazacycloundecine
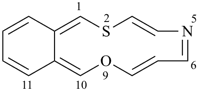
9,2,5-benzoxathiaazacyclododecine (PIN)
(not 2,9,6-benzoxathiaazacyclododecine; the locant set 2,5,9 is lower than 2,6,9)
benzo[j][1,8,5]oxathiaazacyclododecine
P-25.3.1 Definitions, terminology, and general principlesP-25.3.1 Definitions, terminology, and general principles
P-25.3.2 Constructing two-component fusion names
P-25.3.3 Numbering of fused ring systems
P-25.3.4 Constructing polycomponent fusion names
P-25.3.5 Heteromonocyclics fused to a benzene ring
P-25.3.6 Identical attached components
P-25.3.7 Multiparent ring systems
P-25.3.8 Omission of locants in fusion descriptors
P-25.3.1.1 Definitions
P-25.3.1.1.1 ortho-Fused. Two rings that have only two atoms and one bond in common are said to be ortho-fused
Example:
Example:
P-25.3.1.1.4 Peripheral atom. Any atom that forms part of the outer perimeter of a fused ring system.
P-25.3.1.1.5 Interior atom. Any fusion atom that is not peripheral.
P-25.3.1.2 Terminology
P-25.3.1.2.1 Components of a fused ring system. Fusion components are mancude or ring systems that can be named without the application of any fusion nomenclature principles. Fused ring systems that do not have such a name are named by joining together the names of appropriately selected fusion components.
P-25.3.1.2.2 Parent component. The parent component according to the terminology of the 1998 recommendations (ref. 4) (referred to as base component in the 1979 publication, ref. 1; and principal component in the 1993 Recommendations, ref. 2) is the one with highest seniority according to the criteria given in P-25.3.2.4. A parent component may be mono- or polycyclic, but it must be a mancude ring or ring system. Its name is never modified and is cited last in the name of the fused system.
P-25.3.1.2.3 Attached component. The components of a fused ring system not included in the parent component are called attached components. The attached components are called first-order, second-order, etc. attached components when they correspond to the first, second, etc. attached component reached when moving away from the parent component across fusion sites. An attached component may be mono- or polycyclic, but it must be a mancude ring or ring system. Fusion sites are bold lines in the following examples.
Example:
P-25.3.1.2.4 Interparent components. In a system that consists of two (or more) parent components ortho- or ortho- and peri-fused to the same attached component, the latter is called an interparent component. Likewise, if two (or more) parent components are fused to three or more appropriately attached components, there will be two first- order interparent components and a second-order interparent component. Fourth-, fifth-, etc. order components may be present in more complex systems.
Example:
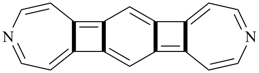
P-25.3.1.3 General principles
‘ortho-Fused’ or ‘ortho- and peri-fused’ polycyclic ring systems with the maximum number of noncumulative double bonds (mancude) that have no accepted retained or systematic name described in sections P-25.1 and P-25.2 are named by prefixing to the name of a component ring or ring system (the parent component) designations of the other component(s) (attached components). For fused ring systems in preferred IUPAC names, see P-52.2.4.
The parent component is selected by applying criteria of seniority as described in P- 25.3.2.4 below. In a fusion name, the name of the parent component is that of the component itself. The names of attached components are formed by replacing the last letter ‘e’ by ‘o’ in the name of the component, i.e., indeno from indene (or by adding the letter ‘o’ when no final letter ‘e’ is present, i.e., pyrano from pyran) or by other means described in P-25.3.2.2 below. There is no elision of the final letter ‘o’ or ‘a’ before a vowel (see Rule FR-4.7, ref. 4).
Locants that describe structural features of components, such as positions of heteroatoms, are kept with the name of the component and are enclosed within square brackets.
Note: In preferred IUPAC names the elision of the final letter ‘o’ of ‘acenaphtho’, ‘benzo’, ‘naphtho’ and ‘perylo’ and the final letter ‘a’ of the monocyclic prefixes ‘cyclopropa’, ‘cyclobuta’, etc. is not recommended as indicated in FR-4.7 of the 1998 publication, ref. 4; hence, ‘benzo[g]isoquinoline’ rather than ‘benz[g]isoquinoline’. However this elision as recommended in the 1979 recommendations (Rule A-21.4, ref. 1) may be continued in general nomenclature.
Isomers are distinguished by lettering, continuously, each peripheral side of the parent component (including sides whose locants are distinguished by letters, for example, 2a,3a) using the italic letters a, b, c, etc., beginning with a for the side numbered ‘1,2’, b for ‘2,3’ etc.. To the letter as early in the alphabet as possible that denotes the side where the fusion occurs are prefixed, if necessary, the numbers of the positions of attachment of the other component. These numbers are chosen to be as low as is consistent with the numbering of the compound and their order conforms to the direction of lettering of the parent component. In this document these letters and numbers are placed within the structure of the ring or ring system.
Examples:
 |  |
| azulene (PIN) + naphthalene (PIN) (parent component) (attached component) | naphtho[1,2-a]azulene (PIN) |
 | 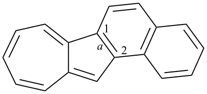 |
| azulene (PIN) + naphthalene (PIN) (parent component) (attached component) | naphtho[2,1-a]azulene (PIN) |
The numbers and letters, separated by commas when required, are enclosed in square brackets and placed immediately after the designation of the attached component; there is no space or hyphen either preceding or following the brackets. Hyphens separate the two parts of a fusion descriptor, i.e., numbers and italicized letters. This expression merely defines the manner of fusion of the components. Indicated hydrogen atoms are added to the names, as required, using locants characterizing the fused system.
Examples:
selenopheno[3,4-b]selenophene (PIN)
selenopheno[3,2-b]selenophene (PIN)
Examples:
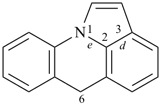
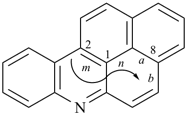
naphtho[2,1,8-mna]acridine
A component may be monocyclic or polycyclic. Systematic construction proceeds stepwise as follows.
P-25.3.2.1 Selecting and naming parent components for fusion nomenclatureP-25.3.2.1 Selecting and naming parent components for fusion nomenclature
P-25.3.2.2 Prefixes for attached components
P-25.3.2.3 Orientation of fused ring systems
P-25.3.2.4 Seniority criteria for selecting parent components
P-25.3.2.5 Assembling components and naming fused ring systems
P-25.3.2.1.1 Monocyclic hydrocarbons (annulenes).
Monocyclic parent components are named as [n]annulenes where n represent the number of carbon atoms. The series starts at n = 7, because the retained name ‘benzene’ is preferred for n = 6. The use of the name ‘annulene’ in fusion nomenclature was recommended in the 1993 Guide (see R-2.3.1.2, ref. 2) to obviate the potential ambiguity of using contracted traditional names, such as cycloheptene to denote 1,3,5-cycloheptatriene.
Examples:
[10]annulene
(no longer cyclodecene as fusion component)
cyclodeca-1,3,5,7,9-pentaene (PIN)
The retained names given in Table 2.2, except for ‘isothiazole’, ‘isoxazole’, ‘thiazole’, and ‘oxazole’, and Hantzsch-Widman names for unsaturated heteromonocycles (see P-22.2.2) are used as parent components in fusion nomenclature. The names ‘isothiazole’, ‘isoxazole’, ‘thiazole’, and ‘oxazole’, although permitted in general nomenclature, are not recommended for the names of components in preferred IUPAC fusion names. The Hantzsch-Widman names 1,2-thiazole, 1,2-oxazole, 1,3- thiazole, and 1,3-oxazole, respectively, must be used; the locants are enclosed in square brackets in the completed fusion name.
Heteromonocycles having more than ten members and the maximum number of noncumulative double bonds whose names are denoted by the ‘ine’ ending described in P-25.2.2.1.2 are used as parent components in preferred IUPAC fusion names.
Examples:
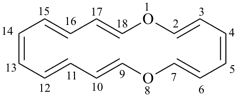
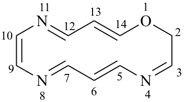
2H-1,4,8,11-oxatriazacyclotetradecine
1-oxa-4,8,11-triazacyclotetradeca-3,5,7,9,11,13-hexaene (PIN)
P-25.3.2.2 Prefixes for attached components.
P-25.3.2.2.1 Monocyclic hydrocarbon prefixes for attached components other than ‘benzo’ are formed by dropping ‘ne’ from the name of the appropriate saturated monocyclic hydrocarbon. These names represent the form with the maximum number of noncumulative double bonds. There is no upper ring-size limit to this criterion.
Examples:


cyclobuta (preferred prefix)
(from cyclobutane, PIN)
cyclopenta (preferred prefix)
(from cyclopentane, PIN)
cyclohepta (preferred prefix)
(from cycloheptane, PIN)
(not [7]annuleno)
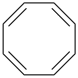
cycloocta (preferred prefix)
(from cyclooctane, PIN)
(not [8]annuleno)
Examples:
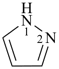
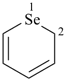
selenopyrano (preferred prefix)
(from selenopyran, PIN)
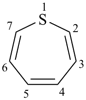
thiepino (preferred prefix)
(from thiepine, PIN)
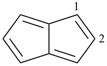
pentaleno (preferred prefix)
(from pentalene, PIN)
1,4,8,11-oxatriazacyclotetradecino (preferred prefix)
(from 1,4,8,11-oxatriazacyclotetradecine, see P-25.2.2.1.2, P-25.3.2.1.2)
Only the following contracted prefixes are retained for preferred IUPAC fusion names. The contracted prefixes acenaphtho, perylo, isoquino, and quino are retained but are only used in general nomenclature.
naphtho (preferred prefix)
(from naphthalene, PIN)
benzo (preferred prefix)
(from benzene, PIN)
phenanthro (preferred prefix)
(from phenanthrene, PIN)
furo (preferred prefix)
(from furan, PIN)
imidazo (preferred prefix)
(from imidazole, PIN)
pyrido (preferred prefix)
(from pyridine, PIN)
pyrimido (preferred prefix)
(from pyrimidine, PIN)
thieno (preferred prefix)
(from thiophene (PIN)
P-25.3.2.3.1 Drawing of ring structures
For the purpose of selecting parent components and for numbering of fused ring systems, the structures of fused ring compounds must be drawn in a specific manner according to a set of criteria that must be applied in order until a decision is reached. Individual rings of a polycyclic ‘ortho-fused’ or ‘ortho- and peri-fused’ hydrocarbon ring system are drawn in such a way so that as many as possible of the various individual rings are arranged in horizontal rows. If the compound requires distorted rings not shown below see P-25.3.2.3.2. Such rows are characterized by a horizontal axis that divides each individual ring into two approximate halves. Permitted shapes for three- to eight-membered rings are as follows:

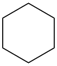
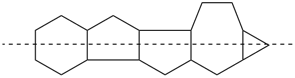
P-25.3.2.3.2 Distorted ring shapes
If a compound cannot be drawn only using the shapes shown in P-25.3.2.3.1 a distorted ring shape will be required. The distorted ring should be as small as possible.
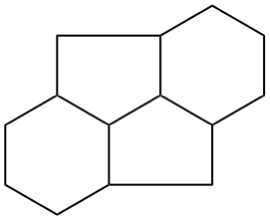 | not | 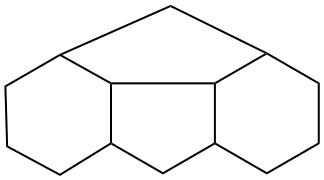 |
| only preferred shapes used | distorted five-membered ring | |
 | not |  |
| only preferred shapes used (the separation to show the seven-membered ring is not fused to the left hand ring does not count as a distortion) | distorted seven-membered ring | |
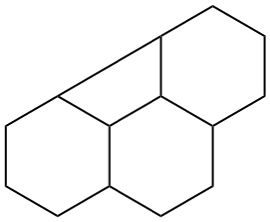 | not |  |
| distorted four-membered ring | distorted six-membered ring |
P-25.3.2.3.3 Criteria for selection of the preferred orientation
Polycyclic fused ring systems are oriented in accordance with the following criteria considered in order until a decision is reached:
(a) maximum number of rings in a horizontal row;
Fused ring systems are drawn in order to achieve the maximum number of ortho-fused rings, with vertical common bonds, in a horizontal row. The relevant vertical bonds are always those furthest apart. If the correct orientation is not immediately apparent the horizontal row is bisected by a horizontal axis and a vertical axis to form four quadrants. Rings which are not bisected by the horizontal axis do not belong to the main row and are not considered in the counting of rings in the main row.
Examples:
2 rings in horizontal row
(b) maximum number of rings in upper right quadrant;
In the preferred orientation, the maximum number of rings must appear above and to the right of the horizontal row (upper right quadrant). For this purpose, the center of the horizontal row is defined as the central common bond if there is an even number of rings in the row, and the center of the central ring if there is an odd number of rings. In counting rings in a quadrant those rings that are divided by an axis are considered as two halves, and a ring bisected by both axes, counts as four quarters (one in each quadrant). Rings that are bisected by the horizontal axis but are not directly ortho-fused to the main row are not considered when counting how many rings are in the horizontal row.
Examples:
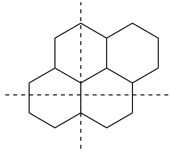 | not | 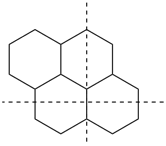 |
| (correct orientation) | (incorrect orientation) | |
| 2 rings in horizontal row 2 rings in upper right quadrant | 2 rings in horizontal row 1 ring in upper right quadrant | |
| : | ||
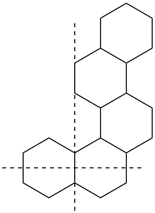 | not | 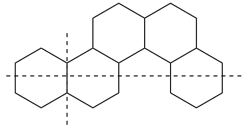 |
| (correct orientation) | (incorrect orientation) | |
| 2 rings in the horizontal row 3½ rings in upper right quadrant | 2 (not 3) rings in the horizontal row 3 rings in upper right quadrant |
Accordingly, phenanthrene (1½ rings in the upper right quadrant) is senior to phenalene [1 ring (two ½ rings) in the upper right quadrant].
(c) minimum number of rings in the lower left quadrant;
Example:
 | not |  |
| (correct orientation) | (incorrect orientation) | |
| 3 rings in horizontal row 1¾ rings in upper right quadrant ¾ ring in lower left quadrant | 3 rings in horizontal row 1¾ ring in upper right quadrant 1¾ ring in lower left quadrant |
(d) maximum number of rings above the horizontal row.
Examples:
 | not |  | not | 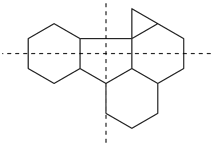 |
| (correct orientation) | (incorrect orientation) | (incorrect orientation) | ||
| 3 rings in horizontal row 1¾ rings in upper right quadrant ¾ ring in lower left quadrant 3½ rings above horizontal row | 3 rings in horizontal row 1¾ ring in upper right quadrant ¾ ring in lower left quadrant 2½ rings above horizontal row | 3 rings in horizontal row 1¾ ring in upper right quadrant ¾ ring in lower left quadrant 2½ rings above horizontal row |
P-25.3.2.4 Seniority criteria for selecting the parent component
The components of the fused ring system are selected and named according to P-25.3.2.1 and P-25.3.2.2. When it is necessary to locate nomenclatural features, such as indicated hydrogen or atoms with nonstandard bonding numbers, another system of locants must be used, i.e., the locants that are used to number the completed fused ring system. In these recommendations, such locants are placed outside the structure, as shown for retained names in Tables 2.2, 2.7, and 2.8. This system is fully explained and exemplified in P-25.3.3.
If there is a choice for selecting the parent component, the following criteria are considered, in order, until a decision can be made:
(a) a component containing at least one of the heteroatoms occurring earlier in the following order: N > F > Cl > Br > I > O > S > Se > Te > P > As > Sb > Bi > Si > Ge > Sn > Pb > B > Al > Ga > In > Tl;
Examples:

1H,18H-naphtho[1,8-rs][1,4,7,10,13,16]hexaoxacyclohenicosine (PIN)
[1,4,7,10,13,16-hexaoxacyclohenicosine (heterocycle) is senior to naphthalene (carbocycle)]
[1]benzopyrano[2,3-c]pyrrole (PIN)
(pyrrole is senior to 1-benzopyran N > O)
chromeno[2,3-c]pyrrole
2H-[1,4]dithiepino[2,3-c]furan (PIN)
(furan is senior to dithiepine; O > S)
Example:
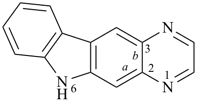
Examples
naphtho[2,3-f]azulene (PIN)
[azulene (7,5 rings) preferred to naphthalene 6,6 rings)]
Examples:
2H-furo[2,3-d][1,3]dioxole (PIN)
[dioxole (2 heteroatoms) preferred to furan (1 heteroatom)]
Example:
Examples:
[1,4]oxaselenino[2,3-b][1,4]oxathiine (PIN) (O,S senior to O,Se)
Examples:
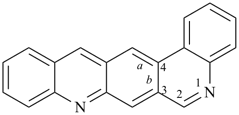
benzo[pqr]tetraphene (PIN)
[tetraphene (3 rings in horizontal row)
preferred to chrysene or pyrene (2 rings in horizontal row)]
Example:
Example:
Examples:
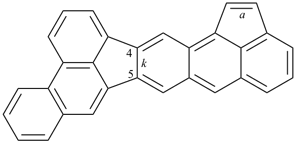
aceanthrylene (PIN)
acephenanthrylene (PIN)
P-25.3.2.5.1 A heteroatom common to two components must be indicated in the name of each component.
Example:
Example:
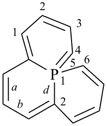
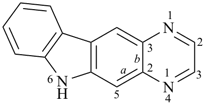
Fused ring systems with retained names, systematic names, or fused names are systematically numbered in the same manner. Anthracene, phenanthrene, acridine, carbazole, xanthene and its chalcogen analogues, purine, and cyclopenta[a]phenanthrene are exceptions; traditional numberings are retained. Two types of numbering are to be considered.
P-25.3.3.1 Numbering of peripheral skeletal atomsP-25.3.3.1 Numbering of peripheral skeletal atoms
P-25.3.3.2 Numbering of interior heteroatoms
P-25.3.3.3 Numbering of interior carbon atoms
P-25.3.3.1.1 The numbering of peripheral atoms in the preferred orientation starts from the uppermost ring. If there is more than one uppermost ring, the ring furthest to the right is chosen. Numbering starts from the nonfused atom most counterclockwise in the ring selected and proceeds in a clockwise direction around the system, including fusion heteroatoms but not fusion carbon atoms. Each fusion carbon atom is given the same number as the immediately preceding nonfusion skeletal atom, modified by a Roman letter ‘a’, ‘b’, ‘c’, ‘d’, etc.
Examples:
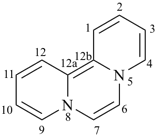
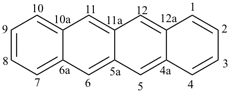
tetracene (PIN)
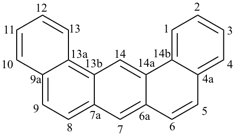
benzo[m]tetraphene (PIN)
Example:
In particular, the orientation and numbering of helicenes has been changed. Recommended numbering and former numbering for hexahelicene are shown below.
Higher helicenes follow the same pattern. A helicene is oriented so that a terminal ring is located in the upper right quadrant; numbering always begins in this terminal ring.
Note: Orientation and numbering no longer recommended;
but still in use by CAS for the ring system having the name phenanthro[3,4-c]phenanthrene, ref. 22)
(a) low locants are assigned to heteroatoms, considered as a set without regard to the kind of heteroatom;
Examples:
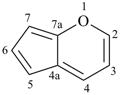
2H,4H-[1,3]oxathiolo[5,4-b]pyrrole (PIN)
Examples:
1H-thieno[2,3-d]imidazole (PIN)
Examples:
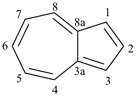 | not | 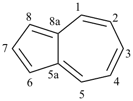 |
| azulene (PIN) | ||
 | not |  | nor | 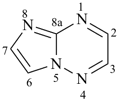 |
| imidazo[1,2-b][1,2,4]triazine (PIN) | ||||
Example:
 | not |  |
| [1,3]diazeto[1,2-a:3,4-a′]bis([1,3]benzimidazole) (PIN) | ||
| [1,3]diazeto[1,2-a:3,4-a′]dibenzimidazole | ||
Example:
 | not | 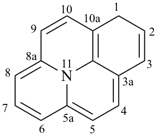 |
| 6H-quinolizino[3,4,5,6-ija]quinoline (PIN) | ||
Examples:
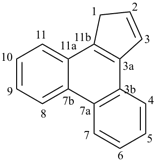
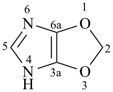 | not | 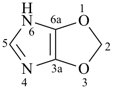 |
| 2H,4H-[1,3]dioxolo[4,5-d]imidazole | 2H,6H-[1,3]dioxolo[4,5-d]imidazole | |
| correct | incorrect |
P-25.3.3.2.1 Interior heteroatoms that are not identified by skeletal replacement (‘a’) nomenclature are numbered after the peripheral atoms continuing the established number sequence [see also P-25.3.3.1.2 (e)]. Compare the numbering of interior carbon atoms (see P-25.3.3.3).
Example:
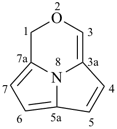
Example:
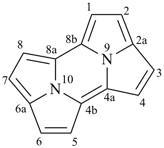
Explanation: The heteroatom numbered ‘9’ is one bond away from ‘2a’, which is lower than ‘4b’.
Example:
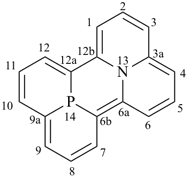
Explanation: The nitrogen atom has a lower locant than the phosphorus atom.
P-25.3.3.3.1 Interior atoms are numbered by identifying the minimum number of bonds linking them to a peripheral atom. The locant for the interior atom is that of the peripheral atom with a superscript number corresponding to the number of bonds between the two atoms. The previous rule (Rule A-22.2 in ref. 1), which is still used in CAS index nomenclature, recommended that interior atoms follow the highest numbered peripheral atom adding Roman letters in sequence to the appropriate peripheral number.
Note: This is a major change to the rules for interior numbering of carbon atoms recommended in the 1998 publication on fusion nomenclature (see FR-5.5.2 in ref. 4).
Examples:
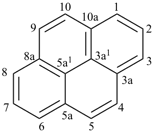 | not | 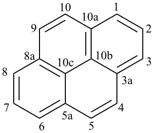 |
| pyrene (PIN) (recommended numbering) | Note: Former numbering, no longer recommended but still used in CAS index nomenclature. | |
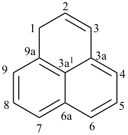 | not | 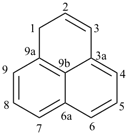 |
| 1H-phenalene (PIN) (recommended numbering) | Note: Former numbering, no longer recommended but still used in CAS index nomenclature. | |
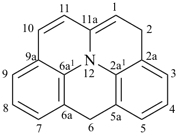 | not | 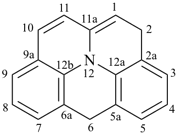 |
| 2H,6H-quinolizino[3,4,5,6,7-defg]acridine (PIN) (recommended numbering) | Note: Former numbering, no longer recommended but still used in CAS index nomenclature. |
Example:
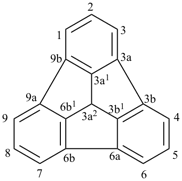 | not | 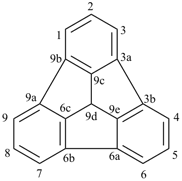 |
| 3a2H-benzo[3,4]pentaleno[2,1,6,5-jklm]fluorene (PIN) (recommended numbering) | Note: Former numbering, no longer recommended but still used in CAS index nomenclature. |
When several nonidentical components must be considered, one, and only one, can be the parent component. All other components are attached components. A component attached directly to the parent component is called a ‘first-order attached component’. A component attached to a first-order attached component is called a ‘second-order’ attached component, and so on. The parent and the first-order attached components are named as indicated for two component systems (see P-25.3.2).
P-25.3.4.1 Three types of fusion names are considered in these recommendations.
P-25.3.4.1.1 Fusion names composed of first- and higher-order attached componentsP-25.3.4.1.1 Fusion names composed of first- and higher-order attached components
P-25.3.4.1.2 Identical attached components
P-25.3.4.1.3 Multiparent names
The procedure for indicating common bond(s) between a first-order attached component and a higher-order attached component follows that for the attachment of the parent compound to the first-order attached component except that numerical locants are used instead of letters and the two sets of locants are separated by a colon. The locants of second-order attached components are primed to contrast with those of first-order attached components. The locants of third-order attached components are doubly primed, and so on.
Example:
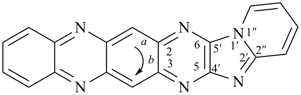
A multiplicity of components that are identical and are all fused to a parent component or an attached component is indicated by the use of the prefix ‘di’, ‘tri’, etc. (or ‘bis’, ‘tris’, etc.). The multiplying prefix is not considered in determining the alphabetical order of attached components in names of polycomponent fusion names (see P-25.3.4.2.3). A colon separates the sets of locants, and a comma is used when letters only are present.
Examples:
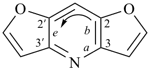
5H-furo[3,2-g]dipyrano[2,3-b:3′,4′,5′-de]quinoline (PIN)
(furo before dipyrano)
Multiple occurrences of the parent component in a multiparent system is indicated by the use of multiplying prefixes, ‘di’, ‘tri’, etc, or ‘bis’, ‘tris’, etc. To distinguish between the parent components, the second has primed locants, the third double primed, etc.
The use of enclosing parentheses following ‘di’, ‘tri’, etc., indicating multiple occurrences of a parent component in these recommendations is a change from the recommendations in the 1998 publication on fusion nomenclature (ref. 4).
Examples:
benzo[1,2-c:3,4-c′]bis([1,2,5]oxadiazole) (PIN)
Polycomponent fusion names are constructed by using specific orders of seniority and rules. They are elaborated as follows.
P-25.3.4.2.1 Order of seniority for selecting parent componentsP-25.3.4.2.1 Order of seniority for selecting parent components
P-25.3.4.2.2 Order of seniority for selecting attached components
P-25.3.4.2.3 Order of citation of fusion prefixes
P-25.3.4.2.4 Order of seniority of locants (letters and numbers)
When there are two or more locations for a parent component in a fused ring structure, the following criteria are applied sequentially until a complete distinction is obtained. In the examples below, the senior location is identified by a solid box and other locations with a dashed box.
The senior location is:
(a) the location that enables the whole ring system to be named by fusion nomenclature, thus excluding names of bridged fused systems;P-25.3.4.2.2 Order of seniority for selecting attached componentsExample:
(b) the location that results in a name that does not require attached components higher than first-order. For didactic purposes only, names of attached components of higher order than first-order are written in bold type; 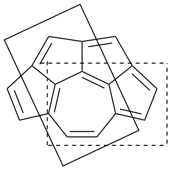
cyclopenta[ij]pentaleno[2,1,6-cde]azulene (PIN)
(not 1,9-methenopentaleno[1,6-ef]azulene
nor 1,9-methenodicyclopenta[cd,f]azulene;
names including the prefix ‘metheno’ are bridged fused ring names)
Example:
(c) the location that results in the maximum number of first-order attached components, second-order attached components, etc. This criterion results in the minimum number of higher-order attached components; hence, fewer primed fusion descriptors are required; 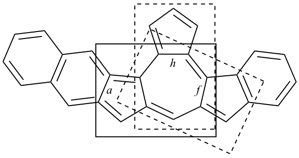
cyclopenta[h]indeno[2,1-f]naphtho[2,3-a]azulene (PIN)
(not benzo[a]benzo[5,6]indeno[2,1-f]cyclopenta[h]azulene
nor benzo[5,6]indeno[1,2-e]indeno[2,1-h]azulene;
there is no second-order component in the recommended name)
Example:
(d) a location that permits the expression of the maximum number of identical attached components with multiplicative prefixes; 
dibenzo[c,g]phenanthrene
(not naphtho[2,1-c]phenanthrene;
two attached components preferred to one)
Example:
(e) a location that uses a senior interparent component; 
dinaphtho[1,2-c :2′,1′-m]picene (PIN)
(not benzo[c]phenanthreno[2,1-m]picene)Example:
(f) a location that results in preferred attached components, first-order, then second-order, etc. This criterion is embellished and illustrated in the original publication (see FR-3.3.1 in ref. 4). 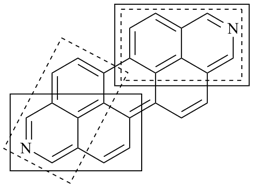
anthra[2,1,9-def:6,5,10-d′e′f′]diisoquinoline (PIN)
(not phenanthro[2,1,10-def:7,8,9-d′e′f′]diisoquinoline;
anthracene is senior to phenanthrene)
After selection of the parent component (see P-25.3.2.4) [or parent components and interparent component(s) if a multiparent name is chosen], other rings are identified as far as possible as attached components. If there is a choice, first-order attached components are considered first, then second-order attached components, etc. The following criteria are applied in order only until a complete decision is made:
Note: In the examples below, the preferred attached component is marked with a solid box and the alternatives with dashed boxes.
(a) if there are alternative first-order attached components, the senior ring or ring system is selected. The same procedure is applied to second-order attached components, and so on;This procedure is continued exploring outwards to the senior second-order attached components.Example:
(b) the location that has lowest locants as a set for fusion of first-order attached components to the parent component; 
8H-cyclopenta[3,4]naphtho[1,2-d][1,3]oxazole (PIN)
(not 8H-benzo[6,7]indeno[5,4-d][1,3]oxazole;
naphthalene is senior to indene)
Example:
(c) the location that has lowest locants for fusion to the parent component in order of citation. 
5H-benzo[6,7]cyclohepta[4′,5′]indeno[1′,2′:3,4]fluoreno[2,1-b]furan (PIN)
(not 5H-benzo[5′,6′]indeno[1′,2′:1,2]cyclohepta[7,8]fluoreno[4,3-b]furan;
locants 1,2 are lower than 3,4 for the ‘fluoreno’ attached component)
Example:
naphtho[2′,1′:3,4]phenanthro[1,2-b]thiophene (PIN)
(not naphtho[2′,1′:3,4]phenanthro[2,1-b]thiophene,
not dibenzo[3,4:5,6]phenanthro[9,10-b]thiophene;
the locants ‘1,2’ for the ‘phenanthro’ attached component, in order of citation, are lower than ‘2,1’ or ‘9,10’)
Example:
P-25.3.4.2.3.1 Fusion between two attached components is indicated by the method described in P-25.3.2. All attached components are cited in front of the parent component. Each second-order attached component is cited in front of the first-order attached component to which it is fused, and so on to higher order attached components. If there are two or more different components, or sets of components, attached to a lower order component, they are cited in alphabetical order.
Examples:
furo[2′,3′:4,5]pyrrolo[2,3-b]imidazo[4,5-e]pyrazine (PIN)
(furo...pyrrolo is cited before imidazo)
5H-furo[3,2-g]dipyrano[2,3-b:3′,4′,5′-de]quinoline (PIN)
(furo is cited before pyrano)
1H-cyclopropa[b]dicyclopenta[2,3:6,7]oxepino[4,5-e]pyridine (PIN)
(not 1H-dicyclopenta[2,3:6,7]oxepino[4,5-b]cyclopropa[e]pyridine;
cyclopropa is cited before dicyclopentaoxepino, which is treated as one component)
Example:
Example:
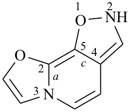
If there is a choice of locants, letters, or numerals (consistent with the numbering of the component), the lower letters or numbers are selected in accordance with the following criteria, which are considered in order until a complete decision can be made:
(a) parent component letters as a set;
Example:
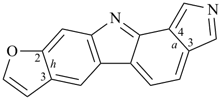
Examples:
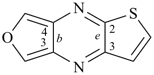
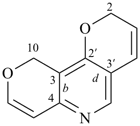
2H,10H-dipyrano[4,3-b:2′,3′-d]pyridine (PIN)
(not 2H,10H-dipyrano[2,3-d:4′,3′-b]pyridine;
b...d is lower than d...b)
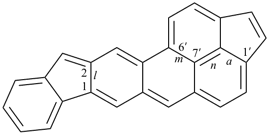
diindeno[1,2-i:6′.7′,1′-mna]anthracene (PIN)
(not diindeno[6,7,1-mna:1′,2′-i]anthracene;
i...mna is lower than mna...i)
Examples:
cyclopenta[1,2-b:5,1-b′]difuran (PIN, a multiparent name)
(not cyclopenta[1,2-b:2,3-b′]difuran;
the locant set ‘1,1,2,5’ is lower than ‘1,2,2,3’)
Example:
Example:
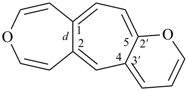
Example:
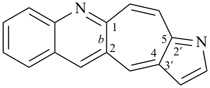
Example:
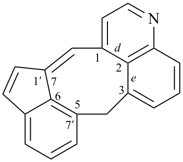
Example:
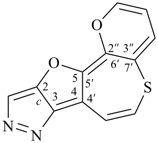
Heterobicyclic compounds consisting of a heteromonocycle fused to a benzene ring in which the benzene ring is not part of a system having a retained name such as quinoline or naphthalene are treated as a single component unit (a ‘benzoheterocycle’, see P-25.2.2.4). They may be treated as a parent component or an attached component depending on the order of seniority described in P-25.3.2.4. However, this approach is not used if it disrupts a multiparent system (see P-25.3.5.3, below) or the use of multiplicative prefixes (see P-25.3.6.1, below).
P-25.3.5.1 A benzoheterocycle as a parent component
Example:
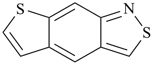
Example:
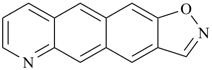
Example:
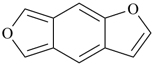
Examples:
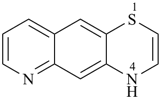
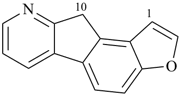
10H-furo[3′,2′:4,5]indeno[2,1-b]pyridine (PIN)
(not 10H-[1]benzofuro[5′,4′:3,4]cyclopenta[1,2-b]pyridine;
pyridine is senior to furan; indene, as a retained name, must be used)
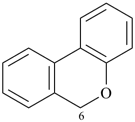
6H-dibenzo[b,d]pyran (PIN)
(not 6H-benzo[c][1]benzopyran;
not 6H-benzo[c][2]benzopyran)
6H-benzo[c]chromene
P-25.3.6.1 Two or more components that are identical and both fused to a parent component are indicated by the use of multiplying prefixes (‘di’, ‘tri’, etc. or ‘bis’, ‘tris’, etc.). If a complete set of locants is used for first-order attached components fused to the parent component, they are cited together separated by a colon. If abbreviated sets of locants are used, the letters are separated by a comma. If complete sets of locants are used for second-order attached components fused to a first-order attached component, or for higher-order cases, the locants are cited together separated by a semicolon. If abbreviated sets are used, they are separated by a colon. To distinguish between two or more components of the same order, the locants of the second component are primed (or double primed if the first is primed, etc.), the third double primed, and so on.
Examples:

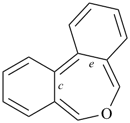
dibenzo[c,e]oxepine (PIN)
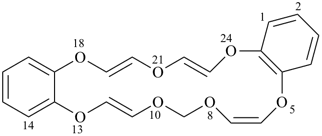
9H-dibenzo[g,p][1,3,6,9,12,15,18]heptaoxacycloicosine (PIN)
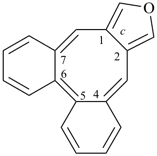
dibenzo[4,5:6,7]cycloocta[1,2-c]furan (PIN)
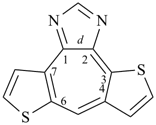
dithieno[2′,3′:3,4;2′′,3′′:6,7]cyclohepta[1,2-d]imidazole (PIN)
cyclopenta[b]dibenzo[3,4:6,7]cyclohepta[1,2-e]pyridine (PIN)
2H,9H-bis([1,3]benzodioxolo)[4,5,6-cd:5′,6′-f]indole (PIN)
Fusion of a higher-order attached component to a system named with a multiplicative prefix requires each set of attached components to be specified separately.
Example:
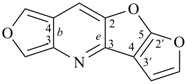
Two or more groups of identical components (including identical fusion locants between these components) fused to another component, are indicated by the use of the multiplying prefixes ‘bis’, ‘tris’, etc., and the group of components is cited within parentheses.
Example:
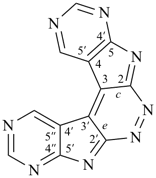
Multiple occurrences of the parent component in a multiparent system is indicated by the use of multiplying prefixes, ‘di’, ‘tri’, etc., or ‘bis’, ‘tris’, etc. To distinguish between the parent components, the second has primed locants, the third double primed, etc.
Two or more nonoverlapping locations for parent components that are ortho- or ortho- and peri-fused to the same first-order interparent component are treated as a multiparent system and given a multiparent name. Similarly a system with three, five, seven, etc. interparent components is treated as an extended multiparent system. Each pair of second- or higher-order interparent components must be identical.
P-25.3.7.1 Multiparent systems with one interparent component
Multiple occurrences of the parent component in a multiparent system are indicated by the use of a multiplying prefix (‘di’, ‘tri’, etc., or ‘bis’, ‘tris’, etc.). To distinguish between the parent components, the second parent component has primed letters, the third double primed, etc.; the sets of locants are separated by a colon.
The use of enclosing parentheses following ‘bis’, ‘tris’, etc. indicating multiple occurrences of a parent component in these recommendations is a change from the recommendations in the 1998 publication on fusion nomenclature (ref. 4).
Examples:
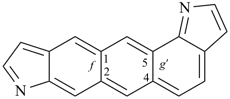
benzo[1,2:4,5]di[7]annulene (PIN)
1H-benzo[1,2:3,4:5,6]tri[7]annulene (PIN)
[1,4,7,10]tetraoxacyclohexadecino[13,12-b:14,15-b′]dipyridine (PIN)
cyclopenta[1,2-b:5,1-b′]bis([1,4]oxathiine) (PIN)
phenanthro[4,5-bcd:1,2-c′]difuran (PIN)
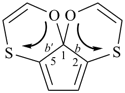
cyclopenta[1,2-b:1,5-b′]bis([1,4]oxathiine) (PIN)

benzo[1,2-c:3,4-c′]bis([1,2,5]oxadiazole) (PIN)
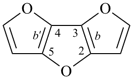
furo[3,2-b:4,5-b′]difuran (PIN, a multiparent name)
[not difuro[3,2-b:2′,3′-d]furan (a multiplicative prefix name);
the multiparent method applies to situations in which there are three identical fused rings or ring systems
according to the principle that a maximum of parent components is preferred to a maximum of attached components]
Additional attached components may be fused to any of the components of a multiparent system. If the choice of locants described in P-25.3.4.2.4 does not permit a final choice, seniority is given to the unprimed component and the fusion letters are assigned with the lower letter used for fusion to the connecting component. Great care is needed with the use of primes, double primes, etc. to ensure that there is no ambiguity. Thus additional components fused to the interparent component(s) are cited in front of the prefix for the interparent component.
Examples:
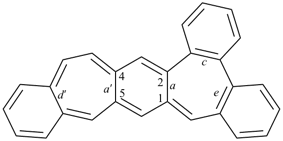
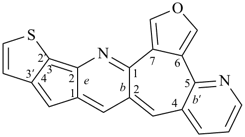
thieno[2′,3′:3,4]cyclopenta[1,2-e]furo[3′,4′:6,7]cyclohepta[1,2-b:5,4-b′]dipyridine (PIN)
(the furan ring is fused to the interparent component; thus, alphabetical order does not apply)
When two (or more) possible parent components are separated by an odd number of interparent components and these are ordered symmetrically with respect to their component rings (but not necessarily with their fusion locants), the whole system is treated as a multiparent system. Names are formed by extending P-25.3.7.1 in two ways as follows:
(a) Second- and higher-order interparent components are named using the multiplying prefixes ‘di’, ‘tri’, etc., or ‘bis’, ‘tris’, etc. Appropriate locants are assigned to interparent components, unprimed and primed for first-order interparent components, double primed for second-order interparent components, tripled primed for third-order interparent components, and so on.For the method used in generating preferred IUPAC names, see P-52.2.4.3.(b) When symmetry permits grouping of interparent components and parent components, such groups can be formed and cited as such using the multiplying prefixes ‘bis’, ‘tris’, etc. to denote groups that are enclosed within parentheses. Unprimed locants only are used within such groupings.
Examples:
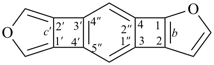
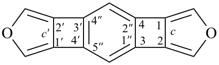
benzo[1′′,2′′:3,4;4′′,5′′:3′,4′]bis(cyclobuta[1,2-c]furan)
P-25.3.8.1 In preferred IUPAC names and in general nomenclature, numerical and/or letter locants are omitted in fused ring systems with only first-order monocyclic attached hydrocarbon components, ‘benzo’, and those described in P-25.3.2.2.1; the omission is also recommended when the fused ring system is made of two monocyclic hydrocarbons.
Examples:
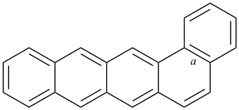
1H-cyclopenta[8]annulene (PIN)
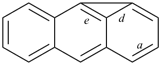
cyclopropa[de]anthracene (PIN)
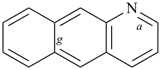
benzo[g]quinoline (PIN)
1H-naphtho[2,3-d][1,2,3]triazole (PIN)
(not 1H-naphtho[2,3][1,2,3]triazole)
Example:
Example:
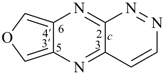
Examples:
quinolizino[4,5,6-bc]quinazoline (PIN)
Example:
This Section is based on the publication ‘Nomenclature of Fused and Bridged Fused Ring Systems’ (ref. 4). Sections P-25.4.2.1.2, P-25.4.2.1.4 and P-25.4.2.2.1 contain modifications to Section FR-8.3 of the 1998 publication (ref. 4). No other modifications have been made to the 1998 publication.
P-25.4.1 Definitions and terminologyP-25.4.1 Definitions and terminology
P-25.4.2 Names of bridges
P-25.4.3 Naming bridged fused ring systems
P-25.4.4 Numbering bridge atoms
P-25.4.5 Order for numbering of bridges
P-25.4.1.1 Bridged fused ring system. A ring system in which some of the rings constitute a fused ring system (see P-25.0 through P-25.3) and the remaining rings are created by one or more bridges.
P-25.4.1.2 Bridge. An atom or group of atoms is named as a bridge by means of a prefix if it fulfills one or more of the following:
(a) if it connects two or more nonadjacent positions of the same ring in a fused ring system;Examples (bridges are indicated in bold):(b) if it connects two or more positions of different rings of a fused ring system and does not thereby form a new ortho- and peri- fused ring;
(c) if it connects positions of a ring of a fused ring system to a previously described bridge but cannot be included as part of that bridge;
(d) if it connects the atoms at the end of a bond common to two rings of a fused ring system;
(e) if it is used to describe a system with only ortho- or ortho- and peri- fusions that cannot be named entirely by fusion principles.
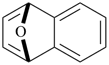
criterion (b)
criterion (c)
criterion (d)
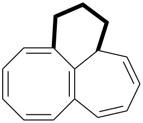
criterion (e)
P-25.4.1.4 Simple bridge A bridge that describes an atom, or groups of atoms, which may be described as a single unit, for example ‘epoxy’, ‘butano’, ‘benzeno’.
P-25.4.1.5 Composite bridge. A group of atoms that can only be described as a contiguous sequence of simple bridges, for example (epoxymethano) = epoxy + methano = –O-CH2–.
P-25.4.1.6 Divalent bridge. A bridge that is connected by single bonds to two different positions of a fused ring system or bridged fused ring system. All bridges described in P-25.4.2.1 are simple bridges.
P-25.4.1.7 Polyvalent bridge. A bridge that is connected to a fused ring system by three or more single bonds or their multiple bond equivalent. Polyvalent bridges may often be described by a combination of two simple divalent bridges. Polyvalent bridges may be further classified as bipodal, tripodal, etc., when the bridge is attached to two, three, or more positions of the fused ring system.
Examples:
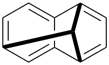
a bipodal trivalent bridge
P-25.4.1.9 Dependent bridge. A bridge that connects one or more positions of a fused ring system to one or more positions of a simple or composite independent bridge, and cannot be expressed as part of a larger composite bridge.
Example:
The extensive list of bridge names given in the publication on fused ring and bridged fused ring nomenclature (ref. 4) has been thoroughly reviewed and updated in the context of the recommendations given herein. Most of the changes occur in the names of acyclic heteroatom bridge names, for which see P-25.4.2.1.4 and P-25.4.2.2.1. For the use of skeletal replacement (‘a’) nomenclature for naming bridges, see P-25.5.1.2.
P-25.4.2.1 Divalent bridges.
P-25.4.2.1.1 A divalent acyclic hydrocarbon bridge is named as a prefix derived from the corresponding unbranched hydrocarbon name by changing the final letter ‘e’ to ‘o’. The locant for a double bond, if present, is indicated in square brackets between the hydrocarbon prefix and the ending ‘eno’; this locant is not the locant used in the final numbering of the bridged fused ring system (see P-25.4.4). If there is a choice, low numbers are preferred (e.g., prop[1]eno rather than prop[2]eno).
Examples:
P-25.4.2.1.2 A divalent monocyclic hydrocarbon bridge other than benzene is named by the same prefix as that used as a fusion prefix (P-25.3.2.2). To distinguish between these two, ‘epi’ is added in front of the name when used as a bridge prefix; the letter ‘i’ in the prefix ‘epi’ is elided before the letters ‘i’ and ‘o’ of the following term. [CAS uses the italicized prefix ‘endo’.] The bridge is assumed to have the maximum number of noncumulative double bonds consistent with its attachments to the fused ring system or to other bridges. The positions of the free valences of the bridge are indicated by locants in square brackets cited directly in front of the bridge name; these locants are not those used for bridge atoms in the final structure, for which see P-25.4.4. Locants for indicated hydrogen atoms, if present, are those for the final structure and are cited in front of the completed name (see P-25.4.3.4).
Examples:
–CH2– methano (preferred prefix) –CH2-CH2– ethano (preferred prefix) –CH2-CH2-CH2– propano (preferred prefix) –CH=CH– etheno (preferred prefix) 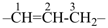
prop[1]eno (preferred prefix) 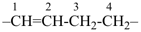
but[1]eno (preferred prefix) 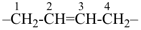
but[2]eno (preferred prefix) 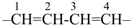
buta[1,3]dieno (preferred prefix)
[1,3]epicyclopropa (preferred prefix)
[‘epicyclopropa’ in ref. 4, FR 8.3.1(b)] 
[1,2]epicyclopenta (preferred prefix)
Examples:
[1,3]benzeno (preferred prefix)
(not [1,3]epibenzo;
benzo is the name of a fusion prefix)
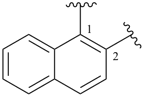
[1,2]naphthaleno (preferred prefix)
(not [1,2]epinaphtho;
naphtho is the name of a fusion prefix)
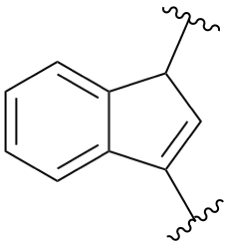
[1,3]epindeno (preferred prefix)
(not [1,3]indeno;
indeno is the name of a fusion prefix)
The following names are now recommended as preselected (see P-12.2) bridge prefixes: ‘sulfano’, –S–;‘disulfano’, –SS–; ‘selano’, –Se–; ‘tellano’, –Te–; ‘azano‘, –NH–; ‘diazeno’, –N=N–; ‘epitriazano’, –NH-NH-NH–; and –NH-N=N–, ‘epitriaz[1]eno’. The bridge prefixes ‘epithio’, ‘epidithio’, ‘episeleno’, ‘epitelluro’, and ‘epimino’ [ref. 4, FR-8.3.1(d)] may be used in general nomenclature, as noted in the following examples.
Examples:
| –O– | epoxy (preselected prefix) (not epoxidano) |
| –O-O– | epidioxy (preselected prefix) (not epiperoxy) |
| –O-O-O– | epitrioxy [preselected prefix, see ref. 4, FR-8.3.1(d)] epitrioxidanediyl |
| –S– | sulfano (preselected prefix) epithio [see ref. 4, FR-8.3.1(d)] |
| –SH2– | λ4-sulfano (preselected prefix) |
| –S-S– | disulfano (preselected prefix) epidithio [see ref. 4, FR-8.3.1(d)] |
| –Se– | selano (preselected prefix) episeleno [see ref. 4, FR-8.3.1(d)] |
| –Te– | tellano (preselected prefix) epitelluro [see ref. 4, FR-8.3.1(d)] |
| –SiH2– | silano (preselected prefix) |
| –SnH2– | stannano (preselected prefix) |
| –NH– | azano (preselected prefix) epimino [see ref. 4, FR-8.3.1(d)] (not imino given in ref. 1, C-815.2) |
| –NH-NH– | diazano (preselected prefix) biimino (see ref. 1, B-15.1) |
| –N=N– | diazeno [preselected prefix; see ref. 4 FR-8.3.1(d)]) azo (see ref. 1, B-15.1) |
| –NH-NH-NH– | epitriazano (preselected prefix) (not triazano, which has been used for H2N-NH-NH–, see C-942.3, ref. 1, which is triazan-1-yl in these recommendations, see P-68.3.1.4.1) |
 | epitriaz[1]eno (preselected prefix) (not triaz[1]eno, which has been used for H2N-N=N–, see C-942.3, ref. 1, which is triaz-1-en-1-yl in these recommendations, see P-68.3.1.4.1) azimino (see ref. 1, B-15.1) |
| –PH– | phosphano (preselected prefix) |
| –BH– | borano (preselected prefix) |
Examples:
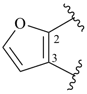
[2,3]furano (preferred prefix)
(not [2,3]epifurano;
nor [2,3]epifuro)
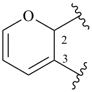
[2,3]epipyrano (preferred prefix)
[3,4]epi[1,2,4]triazolo (preferred prefix)
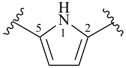
[2,5]epipyrrolo (preferred prefix)
P-25.4.2.2.1 A polyvalent bridge consisting of one atom (other than hydrogen) is named as a prefix based either on a substitutive prefix to which the prefix ‘epi’ is added as described in P-25.4.2.1.4 or by changing the ending ‘ano’ in the name of a divalent monoatomic bridge described in P-25.4.2.1.4 into ‘eno’, for example ‘metheno’ from ‘methano’. Polyvalent bridges are enclosed within parentheses in names of bridged fused systems; as a reminder, parentheses are placed around the names of the bridges themselves in the examples below. Where there is a choice, an attachment by a single bond, i.e., ‘yl’, is to the lower locant of a parent ring system. For ‘epi’, see P-25.4.2.1.4.
Examples:
| –CH= | (metheno) (preferred prefix) (epimethanylylidene) |
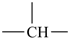 | (epimethanetriyl) (preferred prefix) (methyno) |
 | (epimethanediylylidene) (preferred prefix) |
| –N= | (azeno) (preselected prefix) (epiazanylylidene) (not ‘nitrilo’, see ref. 2, R-9.2.2) |
 | (episilanetetrayl) (preselected prefix) |
 | (epinitrilo) (preselected prefix) epiazanetriyl [ref. 4, FR-8.3.2 (a)] [not nitrilo] |
| –P= | (phospheno) (preselected prefix) (epiphosphanylylidene) [ref. 4, FR-8.3.2 (a)] |
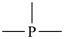 | (epiphosphanetriyl) (preselected prefix) [not phosphanetriyl, [ref. 4 FR-8.3.2 (a); nor phosphinidyne] |
Examples:
| –CH2-CH= | (epiethanylylidene) (preferred prefix) |
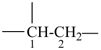 | (epiethane[1,1,2]triyl) (preferred prefix) |
 | (epiethene[1,1,2]triyl) (preferred prefix) |
 | (epibuta[1,3]diene[1,1,4]triyl) (preferred prefix) |
 | (epibut[3]ene[1,1,2,4]tetrayl) (preferred prefix) |
| =N-N= | (epidiazanediylidene) (preselected prefix) |
 | (epibenzene[1,2,3,4]tetrayl) (preferred prefix) |
P-25.4.2.3.1 Composite bridge names are formed by concatenating the names of two or more simple bridges. Unless cited first, the prefix ‘epi’ (or ‘ep’ before the letter ‘i’ or ‘o’ of the following term) is omitted. The prefixes are cited without elision in order starting from the senior prefix based on the following criteria applied in turn until a decision is reached.
(a) the simple bridge containing the heteroatom appearing first in the list: O > S > Se > Te > N > P > As > Sb > Bi > Si > Ge > Sn > Pb > B > Al > Ga > In > Tl;P-25.4.2.3.2 Composite bridges are enclosed within parentheses in names of bridged fused compounds (as a reminder, parentheses are placed around the names of bridges themselves in the examples below). Locants for indicated hydrogen atoms required for a cyclic component of a composite bridge are cited in front of the name of the complete bridged fused ring system and not in front of the name of the bridge itself (see P-25.7.1). For numbering of bridges see P-25.4.4. If an acyclic bridge component requires internal numbering it is numbered in the direction implied by the bridge name.(b) the simple bridge containing the senior ring system;
(c) the simple bridge containing the longer acyclic chain;
(d) the simple bridge that occurs first in alphanumerical order.
Examples:
| –O-CH2– | (epoxymethano) (preferred prefix) |
| –NH-CH2-CH2– | (azanoethano) (preferred prefix) |
| –O-S-O– | (epoxysulfanooxy) (preselected prefix) |
 | (epoxyethane[1,1,2]triyl) (preferred prefix) |
| –O-CH2-CH< | (epoxyethane[1,2,2]triyl) (preferred prefix) |
 | ([1,4]benzenomethano) (preferred prefix) |
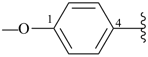 | (epoxy[1,4]benzeno) (preferred prefix) |
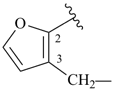 | ([2,3]furanomethano) (preferred prefix) |
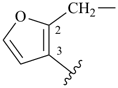 | ([3,2]furanomethano) (preferred prefix) |
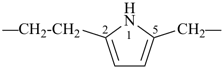 | [ethano([2,5]epipyrrolo)methano] (preferred prefix) |
| –CH2-O-CH= | (methanooxymetheno) (preferred prefix) |
P-25.4.3 Naming bridged fused ring systems
P-25.4.3.1 Citation of bridgesP-25.4.3.1 Citation of bridges
P-25.4.3.2 Attachment locants
P-25.4.3.3 Choice of attachment locants
P-25.4.3.4 Selection of bridges
If there is more than one bridge, they are cited in alphabetical order, unless one bridge is dependent on another. In that case, the dependent bridge is cited in front of all independent bridges. Two or more identical bridges are indicated by the numerical prefixes ‘di’, ‘tri’, etc. with simple bridges (P-25.4.1.4), or ‘bis’, ‘tris’, etc. with composite bridges (P-25.4.1.5) or if ‘di’, ‘tri’, etc. would be ambiguous. The locant pairs of identical bridges are separated by colons.
P-25.4.3.2 Attachment locants
The fused ring system to be bridged is numbered in the usual way. For selection of the fused ring to be bridged see P-25.4.3.4.2.
P-25.4.3.2.1 Divalent symmetric bridges
The locants of the positions on the fused ring system to which divalent symmetric bridges (are) attached are cited in numerical order in front of each bridge name.
Examples:
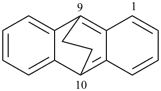
1,4:6,9-dimethanooxanthrene (PIN)
1,4:6,9-dimethanodibenzo[b,e][1,4]dioxine
6,9-epoxy-1,4-methanobenzo[8]annulene (PIN)
1H-1,4-ethanothioxanthene (PIN)
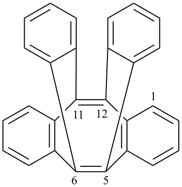
5,12:6,11-di[1,2]benzenodibenzo[a,e][8]annulene (PIN)
Locants of the positions on the fused ring system to which polyvalent or composite bridges are attached are cited in the order expressed or implied by the name of the bridge. Methods of assigning locants for the free valences of bridges are given in section P-25.4.2. In the absence of specific locants, a single free valence (yl) is assigned a lower locant than a double free valence (ylidene). If there is a choice, locants are cited in numerical order. Indicated hydrogen atoms are cited at the front of the complete bridged fused ring name and not in front of names of the bridges themselves (P-25.7.1).
Examples:
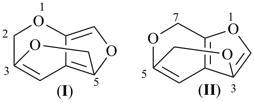
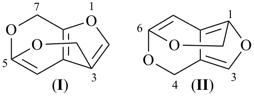
7H-5,3-(epoxymethano)furo[2,3-c]pyran (I) (PIN)
(not 4H-6,1-(epoxymethano)furo[3,4-c]pyran (II)
[preferred ring system, see P-25.4.3.4.2 (d)]
10,5-[2,3]furanobenzo[g]quinoline (PIN)
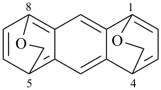
1,4:8,5-bis(epoxymethano)anthracene (PIN)
If there is a choice of attachment locants after the application of P-25.4.3.2, seniority is established in the following order:
(a) the lowest set of locants for all the bridge attachment points considered as a set;
Examples:
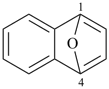
5,8-epoxy-1,3-methanoanthracene (PIN)
(not 1,4-epoxy-5,7-methanoanthracene;
the locant set 1,3,5,8 is lower than 1,4,5,7)
Examples:
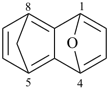
1,4-epoxy-5,8-methanonaphthalene (PIN)
When a polycyclic ring system cannot be named completely as a fused ring system, possible ways for naming it as a bridged fused system are considered. Bridges are selected so that a recommended fused ring system as described in P-25.1 through P-25.3 is the parent fused ring system that is bridged.
P-25.4.3.4.1 Naming of ortho- or ortho- and peri-fused systems
The ring system that remains after removal of the bridge(s) is named according to P-25.1 through P-25.3. The maximum number of noncumulative double bonds is assigned to the fused ring system after the insertion of the bridge. Thus, in allowing for the bonds of the bridge, the number of noncumulative double bonds and/or the need for indicated hydrogen may be different than in the parent ring system. If needed, indicated hydrogen is used to identify the specific isomer and is cited in front of the completed bridged fused ring name (see P-25.7.1.3.2).
Examples:
9H-9,10-ethanoacridine (PIN)
1,5-methanoindole (PIN)
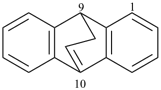
9H-9,10-(epiethanylylidene)anthracene
[not 9H-10,9-(epiethanylylidene)anthracene;
the ‘yl’ suffix is given preference for the low locant
of attachment to the ring system (see P-25.4.3.2.2)]
If there is a choice for selecting the fused ring system to be bridged, the criteria in the following list are applied in order until a complete decision is reached. Two structures might differ in the distribution of double bonds as described in P-25.4.3.4.1. Locants of the fused ring system where bridges are attached are then cited in front of the name of the bridge and in the same order as the locants for the free valences of the bridge (see P-25.4.3.2.2). The fused ring system, before bridging must:
(a) contain the maximum number of rings;
Examples:
 | not |  |
| (I) | (II) | |
| 1H-1,3-propanocyclobuta[a]indene (I) (PIN) [not 8,10,1-(epiethane[1,1,2]triyl)benzo[8]annulene(II) (for order of ring attachment locants see P-25.4.3.2.2) | ||
Explanation: the correct name has three rings in the fused ring system; the incorrect name has only two rings in the fused ring system.
Example:
 |  |  |
| (I) | (II) | (III) |
| 6,7-(epiprop[1]en[1]yl[3]ylidene)benzo[a]cyclohepta[e][8]annulene (I) (PIN) [not 7,6-(epiprop[1]en[1]yl[3]ylidene)benzo[a]cyclohepta[e][8]annulene (II); the ‘yl’ suffix is given the lower attachment point of the ring system (see P-25.4.3.2.2) [not 4,5-buta[1,3]dienodibenzo[a,d][8]annulene (III); the fused ring system benzo[a]cyclohepta[e][8]annulene has 17 atoms whereas the fused ring system dibenzo[a,d][8]annulene has only 16 atoms] | ||
(c) have the fewer heteroatoms in the fused ring system before bridging;
Example:
 | not |  |
| (I) | (II) | |
| 1,4:8,5-bis(epoxymethano)anthracene (I) (PIN) | [not 1H,3H-1,4:6,9-bisethenobenzo[1,2-c:5,4-c′]dipyran (II); | |
| zero heteroatoms in the fused ring system before bridging is fewer than two] | ||
(d) consist of the most senior ring system, when the seniority order is applied to the parent fused ring system (see P-44.2);
Examples:
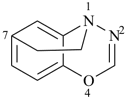 | not | 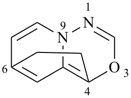 |
| (I) | (II) | |
| 1,7-ethano[4,1,2]benzoxadiazine (I) (PIN) | [not 4,6-ethanopyrido[1,2-d][1,3,4]oxadiazine (II); | |
| the locant set ‘1,2,4’ for the heteroatoms in (I) is lower than the locant set ‘1,3,9’ in (II) (see P-14.3.5)] | ||
 |  |
| (I) | (II) |
| 1,12-ethenobenzo[4,5]cyclohepta[1,2,3-de]naphthalene (I) (PIN) {not 1,12-ethenobenzo[c]phenanthrene (II); the ring size set ‘7,6,6,6’ in (I) is preferred to the ring size set ‘6,6,6,6’ in (II) [see P-25.3.2.4 (c) and P-44.2.2.2.3 (a)]} | |
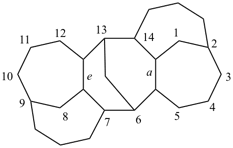 | not | 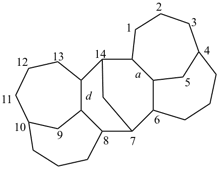 |
| (I) | (II) | |
| octadecahydro-6,13-methano-2,14:7,9-dipropanodicyclohepta[a,e][8]annulene (I) (PIN) {not octadecahydro-7,14-methano-4,6:8,10-dipropanodicyclohepta[a,d][8]annulene (II); in the correct name a greater number of rings of the fusion ring system to be bridged are in a horizontal row, ‘3’ versus ‘2’ [see P-25.3.2.3.2 (a) and P-44.2.2.2.3 (b)]} hexacyclo[15.3.2.23,7.12,12.013,21.011,25]pentacosane (see P-23.2.6.3) | ||
(e) have the minimum number of polyvalent bridges;
Examples:
 | not |  |
| (I) | (II) | |
| 2,6:5,7-dimethanoindeno[7,1-bc]furan (I) (PIN) (for the order of ring attachment locants see P-25.4.3.2.2) [not 5,7,2-(epiethane[1,1,2]triyl)indeno[7,1-bc]furan (II); the PIN (I) has no polyvalent bridges; the incorrect name (II) has one polyvalent bridge] | ||
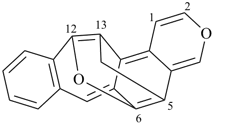 | not | 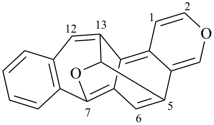 |
| (I) | (II) | |
| 6,12-epoxy-5,13-methanobenzo[4,5]cyclohepta[1,2-f][2]benzopyran (I) (PIN) 6,12-epoxy-5,13-methanobenzo[4,5]cyclohepta[1,2-f]isochromene (I) (for the order of ring attachment locants see P-25.4.3.2.2) [not 7,5,13-(epoxymethanetriyl)benzo[4,5]cyclohepta[1,2-f]isochromene (II); the PIN (I) has no polyvalent bridges; the incorrect name (II) has one polyvalent bridge] | ||
(f) have the minimum number of dependent bridges;
Example:
 | not |  |
| (I) | (II) | |
| 6,11-buta[1,3]dieno-3,8-phosphano[1,4]diazocino[2,3-g]cinnoline (I) (PIN) [not 3,15-phosphano-6,11-buta[1,3]dieno[1,4]diazocino[2,3-g]cinnoline (II); the PIN (I) has no dependent bridges; the incorrect name (II) has one dependent bridge] | ||
(g) have the minimum number of atoms in dependent bridges;
Example:
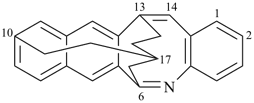
 | not |  |
| (I) | (II) | |
| 8,7-(azenoepietheno)cyclohepta[4,5]cycloocta[1,2-b]pyridine (I) (PIN) [not 8,7-(azenoepiethanediylidene)cyclohepta[4,5]cycloocta[1,2-b]pyridine (II); the PIN has a composite bridge consisting of a trivalent bridge and a divalent bridge whereas the incorrect name contains a composite bridge consisting of a trivalent bridge and a tetravalent bridge] | ||
(i) have the lowest locants at the location of bridges, first for independent bridges then dependent bridges;
Example:
14,5-(metheno)-3,2,4-(epiprop[2]en[1,3]diyl[1]ylidene)dicyclopenta[f,f′]pentaleno[1,2-a:6,5-a′]dipentalene (PIN)
the locants set for the independent bridge ‘3,2,4’ is the lowest; the locant for single bond attachment of metheno bridge is cited first.
[not 5,14-(metheno)-3,4,2-(epipropan[1]yl[1,3]diylidene)dicyclopenta[f,f′]]pentaleno[1,2-a:6,5-a′]dipentalene;
not 14,5-(metheno)-4,2,3-(epiprop[2]en[1,3]diyl[1]ylidene)dicyclopenta[f,f′]]pentaleno[1,2-a:6,5-a′]dipentalene;
not 5,14-(metheno)-4,3,2-(epipropan[1]yl[1,3]diylidene)dicyclopenta[f,f′]]pentaleno[1,2-a:6,5-a′]dipentalene;
(the locants ‘3,2,4’ for independent bridge is lower than ‘3,4,2’, ‘4,2,3’ and ‘4,3,2’]
Example:
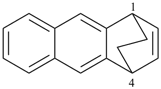
Bridge atoms are numbered continuing from the highest locant of the fused ring system. If there is more than one bridge atom (excluding hydrogen) the numbering starts at the end of the chain or ring atom connected to the bridgehead of the fused ring system having the highest locant. With composite bridges, each component is completely numbered before the next component. Fusion atoms in fused ring bridges are numbered in accordance with P-25.3.3.1.1.
Example:
(a) low locants for heteroatoms;The remaining atoms (excluding hydrogen) are then numbered consecutively.(b) low locants for bridgehead atoms within a bridge;
Examples:
10,5-[2,3]furanobenzo[g]quinoline (PIN)
12H-5,10-[2,5]epipyranobenzo[g]quinoline (PIN)
6,13-(methano[1,2]benzenomethano)pentacene (PIN)
6b,12b-[1,8]naphthalenoacenaphthyleno[1,2-a]acenaphthylene (PIN)
Note: Locants 13a and 17a are generated in the same way as fusion locants for nonbridged fused ring systems, see P-25.3.3.1.1.
P-25.4.5.1 Independent bridges are numbered before dependent bridges.
Example:
Examples
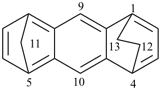
6,14:7,14-dimethanobenzo[7,8]cycloundeca[1,2-b]pyridine (PIN)
Example:
The fusion principles described in P-25.1 through P-25.3 apply only between pairs of components. It is not possible by these principles to name a system in which a third component is ortho- and peri-fused to two components that are themselves ortho- or ortho- and peri-fused together. It is important to recall that benzo heterocycles are considered as one component, thus permitting the construction of names that would not otherwise be possible.
P-25.5.1 Skeletal replacement (‘a’) nomenclature
P-25.5.1.1 If the corresponding hydrocarbon fused ring system can be named by fusion principles or has a retained name, then heteroatoms are identified by skeletal replacement (‘a’) nomenclature using the appropriate ‘a’ prefixes (see P-22.2.3). The numbering of the fused hydrocarbon system is not altered by the ‘a’ prefixes.
When the fusion principles discussed in P-25.1 through P-25.3 do apply, no skeletal replacement (‘a’) name is recommended. The procedure is valid only for cases described in P-25.5.
Examples:
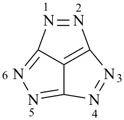
1,3a1,4,9-tetraazaphenalene (PIN)
5H,12H-2,3,4a,7a,9,10,11a,14a-octaazadicyclopenta[ij:i′j′]benzo[1,2-f:4,5-f′]diazulene (PIN)
Examples:
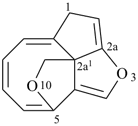
1H-3,10-dioxa-2a1,5-ethanocycloocta[cd]pentalene (PIN)
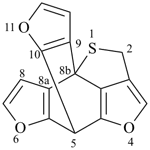
2H,5H-4,6,11-trioxa-1-thia-5,8b-[1,2]epicyclopentacyclopenta[cd]-s-indacene (PIN)
A less preferred hydrocarbon parent component is selected that will permit a fusion name. Second and third choice parent components are chosen according to the seniority order for selecting the senior parent component (see P-25.3.2.4).
Examples:
10-azacyclobuta[1,7]indeno[5,6-b]anthracene (PIN)
[neither quinoline nor pyridine can be used as the senior parent component
because neither naphthalene nor
anthracene, respectively, can be used as the senior attached component;
therefore ‘a’ nomenclature must be used (see P-25.5.1) and
since the preferred hydrocarbon tetracene cannot be used,
the next senior component, anthracene, is chosen as the parent component]
A bridged fused system (see Section P-25.4) is used to generate names for structures that cannot be named by normal fusion nomenclature. A fused system is first envisaged; additional rings are created by using bridges.
Examples:
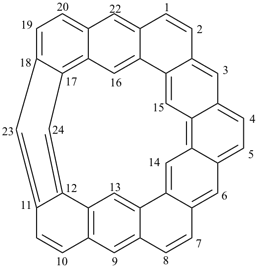 |  | 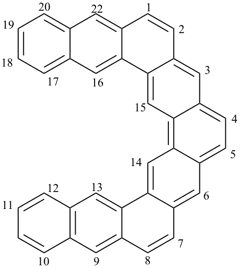 |
| 12,19:13,18-di(metheno)dinaphtho[2,3-a:2′,3′-o]pentaphene (PIN) | ||
The λ-convention is used to describe atoms with nonstandard bonding numbers (ref. 13) in fused ring systems. The symbol λn is used, where ‘n’ is the bonding number of the atoms; it is cited immediately after the locant denoting the atom having the nonstandard bonding number. The symbol H, denoting indicated hydrogen atom(s) with the appropriate locant(s), if present, is cited at the front of the complete name.
The λ symbol is used with all ring systems described in this section with retained and fusion names, bi- and polyalicyclic as well as heterocycles formed by skeletal replacement (‘a’) nomenclature, as described above in P-25.5.1. Atoms with nonstandard bonding numbers in fused ring systems are indicated only in the complete ring system, not in component rings. Names and numbering are unchanged, unless a choice must be made between two otherwise equivalent atoms; in which case, low locants are attributed to atoms with the higher bonding numbers, for example λ6 before λ4.
Examples:
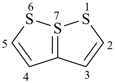
2H-5λ5-phosphinino[3,2-b]pyran (PIN)
1λ4,5-benzodithiepine (PIN)
Examples:
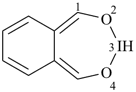
3H-3λ3,2,4-benziodadioxepine (PIN)
3H-2λ4-cyclohepta[c]thiopyran (PIN)
The treatment of double bonds is formalized in fusion nomenclature. A maximum of noncumulative double bonds must be present in fused and bridged fused systems. When hydrogen atoms are in excess at specific positions, they are denoted in names by indicated hydrogen atom(s). However, the presence of cumulative formal double bonds is also possible. This feature is expressed by the δ-convention (delta-Convention) (see ref. 24). These two aspects of fused and bridged fused systems are described in this Section.
P-25.7.1 Indicated hydrogen
P-25.7.1.1 Maximum number of noncumulative double bonds
The names of polycyclic fused ring systems are considered to correspond to the structure with the maximum number of noncumulative double bonds consistent with the appropriate bonding number of the skeletal atoms. To achieve this result, components are fused together and noncumulative double bonds are introduced into the completed fused system. Hydrogen atoms not attached to atoms connected by double bonds are denoted as indicated hydrogen atom(s).
Examples:

2H-1,3-benzoxathiole (PIN)
Example:
Examples:
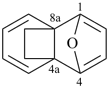
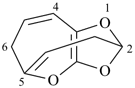
2H,6H-2,5-(epiethanylylidene)[1,3]dioxolo[4,5-b]oxepine (PIN)
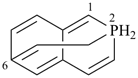
2H-2λ5-2,6-(epiethanylylidene)isophosphinoline (PIN)
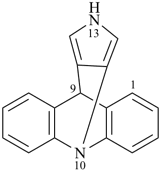
9H,13H-9,10-[3,4]epipyrroloacridine (PIN)
If it is necessary to identify isomers that differ only by virtue of the location of localized double bonds, this differentiation is indicated by the use of the Greek letter Δ. The locant cited corresponds to the first cited locant of the localized double bond.
Examples:
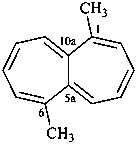
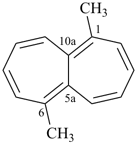
1,6-dimethyl-Δ1(10a)-heptalene (PIN)
P-25.7.1.3.1 When a name applies equally to two or more isomeric systems with the maximum number of noncumulative double bonds and when the name can be made specific by indicating the position of one or more hydrogen atoms in the structure, this specifically is accomplished by adding to the name the italicized symbol H for each of these hydrogen atoms, preceded by the appropriate locant(s).
In preferred IUPAC names, all indicated hydrogen atoms must be cited when the names are constructed in accordance with the principles of fusion nomenclature
In general IUPAC nomenclature, omission of indicated hydrogen atoms is permitted in some parent fused ring systems, when unsubstituted, for example indene rather than 1H-indene, but 1H-indene-3-carboxylic acid. Omission of indicated hydrogen in general nomenclature is permitted in the following ring systems:
| fluorene | 9H |
| indene | 1H |
| phenalene | 1H |
| indazole | 1H |
| indole | 1H |
| isochromene (and chalcogen analogues) | 1H |
| isoindole | 2H |
| perimidine | 1H |
| purine | 7H |
| pyrrole | 1H |
| xanthene | 9H |
Omission of indicated hydrogen is also permitted in general nomenclature if no ambiguity would result, for example 1,3-benzodioxole, rather than 2H-1,3-benzodioxole.
P-25.7.1.3.2 Indicated hydrogen with ortho-, and ortho and peri-fused systems
Indicated hydrogen is identified by the locant of the relevant position and cited at the front of the names of the whole ring system, including replacement terms, if any.
Examples:
6H-1,7-dioxacyclopenta[cd]indene (PIN)
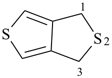
1H,3H-thieno[3,4-c]thiophene (PIN)
All indicated hydrogen atoms are indicated at the front of the complete name.
Note: Citation of indicated hydrogen in bridged fused ring systems in this manner is completely consistent with its citation for spiro atoms (see P-24.3.2) and ring assemblies (see P-28.2.3).
Examples:
1H-3a,7-ethanoazulene (PIN)
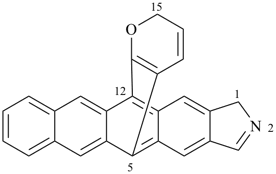
1H,15H-12,5-[2,3]epipyranoanthra[2,3-f]isoindole (PIN)
The presence of contiguous formal double bonds at a skeletal atom in a cyclic parent hydride whose name normally implies the maximum number of noncumulative double bonds is described by the symbol δc, where ‘c’ is the number of double bonds directly attached to the identified atom (see ref. 24). The δc symbol is cited immediately after an expressed locant for the skeletal atom in the name of the compound and follows the λn symbol, if present.
Examples:
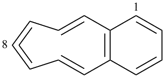
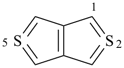
2λ4δ2,5λ4δ2-thieno[3,4-c]thiophene (PIN)
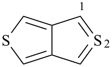
2λ4δ2-thieno[3,4-c]thiophene (PIN)
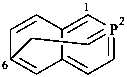
2λ5δ2-6,2-(epiethanylylidene)isophosphinoline (PIN)
In this subsection rings and ring systems are listed in decreasing order of seniority for selection as a parent component. The first list contains heterocyclic parent components, the second hydrocarbon parent components.
P-25.8.1 Partial list of heterocyclic parent components in decreasing order of seniority
The following list contains parent heterocycles arranged in decreasing order of seniority for selection as the parent component for fusion names. Ring systems containing Hg as given in ref. 4 are not included in these recommendations.
The parent heterocycles are arranged by ring analysis, i.e., in decreasing order of number rings, ring size, and in accordance with the priority given to heteroatoms, N, O, S, Se.
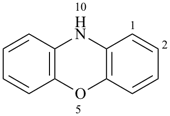
Note 1: Indicated hydrogen atoms are not shown in this kind of listing.Note 2: The seniority order given below is used to select parent components in fusion nomenclature.
Note 3: In the order of seniority used by CAS quinolizine precedes quinoline and isoquinoline; and indolizine preceeds indole and isoindole..
| phenoxazine | C4NO-C6-C6 | |
| phenothiazine | C4NS-C6-C6 | |
| phenoselenazine | C4NSe-C6-C6 | |
| phenotellurazine | C4NTe-C6-C6 | |
| phenazaphosphinine | C4NP-C6-C6 | |
| phenazarsinine | C4NAs-C6-C6 | |
| phenazine | C4N2-C6-C6 | |
| phenanthroline | C5N-C5N-C6 | (in accordance with the positions of nitrogen atoms, in decreasing order of seniority: 1,7; 1,8; 1,9; 1,10; 2,7; 2,8; 2,9; 3,7; 3,8; 4,7) |
| perimidine | C4N2-C6-C6 | |
| acridine | C5N-C6-C6 | |
| phenanthridine | C5N-C6-C6 | |
| carbazole | C4N-C6-C6 | |
| pteridine | C4N2-C4N2 | |
| cinnoline | C4N2-C6 | |
| quinazoline | C4N2-C6 | |
| quinoxaline | C4N2-C6 | |
| 1,5-naphthyridine | C5N-C5N | |
| 1,6-naphthyridine | C5N-C5N | |
| 1,7-naphthyridine | C5N-C5N | |
| 1,8-naphthyridine | C5N-C5N | |
| phthalazine | C5N-C6 | |
| 2,6-naphthyridine | C5N-C5N | |
| 2,7-naphthyridine | C5N-C5N | |
| quinoline | C5N-C6 | |
| isoquinoline | C5N-C6 | |
| quinolizine | C5N-C5N | |
| purine | C3N2-C4N2 | |
| indazole | C3N2-C6 | |
| indole | C4N-C6 | |
| isoindole | C4N-C6 | |
| indolizine | C4N-C5N | |
| pyrrolizine | C4N-C4N |
| pyridazine | C4N2 |
| pyrimidine | C4N2 |
| pyrazine | C4N2 |
| pyridine | C5N |
| pyrazole | C3N2 |
| imidazole | C3N2 |
| pyrrole | C4N |
| phenoxathiine | C4OS-C6-C6 |
| phenoxaselenine | C4OSe-C6-C6 |
| phenoxatellurine | C4OTe-C6-C6 |
| phenoxaphosphinine | C4OP-C6-C6 |
| phenoxarsinine | C4OAs-C6-C6 |
| phenoxastibinine | C4OSb-C6-C6 |
| oxanthrene | C4O2-C6-C6 |
| xanthene | C5O-C6-C6 |
| 1-benzopyran | C5O-C6 |
| 2-benzopyran | C5O-C6 |
| pyran | C5O |
| furan | C4O |
| phenothiarsinine | C4SAs-C6-C6 |
| thianthrene | C4S2-C6-C6 |
| thioxanthene | C4S-C6-C6 |
| 1-benzothiopyran | C5S-C6 |
| 2-benzothiopyran | C5S-C6 |
| thiophene | C4S |
| selenanthrene | C4Se2-C6-C6 |
| selenoxanthene | C5Se-C6-C6 |
| 1-benzoselenopyran | C5Se-C6 |
| 2-benzoselenopyran | C5Se-C6 |
| selenophene | C4Se |
| telluranthrene | C4Te2-C6-C6 |
| telluroxanthene | C5Te-C6-C6 |
| 1-benzotelluropyran | C5Te-C6 |
| 2-benzotelluropyran | C5Te-C6 |
| tellurophene | C4Te |
| phosphanthrene | C4P2-C6 |
| acridophosphine | C5P-C6-C6 |
| phosphanthridine | C5P-C6-C6 |
| phosphinoline | C5P-C6-C6 |
| isophosphinoline | C5P-C6 |
| phosphinolizine | C5P-C6 |
| phosphindole | C4P-C6 |
| isophosphindole | C4P-C6 |
| phosphindolizine | C4P-C5P |
| arsanthrene | C4As2-C6 |
| acridarsine | C5As-C6-C6 |
| arsanthridine | C5As-C6-C6 |
| arsinoline | C5As-C6-C6 |
| isoarsinoline | C5As-C6 |
| arsinolizine | C5As-C6 |
| arsindole | C4As-C6 |
| isoarsindole | C4As-C6 |
| arsindolizine | C4As-C5As |
| silanthrene | C4Si2-C6-C6 |
| boranthrene | C4B2-C6-C6 |
P-25.8.2 Partial list of hydrocarbon parent components in decreasing order of seniority
Parent components are arranged:
(1) in decreasing order of number of rings;
(2) in decreasing order of ring size;
(3) in decreasing order of senior orientation;
(4) for aceanthrylene and acephenanthrylene, in increasing order of fusion atom locants;
(5) in the order of seniority used by CAS the indacenes precede biphenylene.
| ovalene | C6C6C6C6C6C6C6C6C6C6 |
| octaphenylene | C16C6C6C6C6C6C6C6C6 |
| tetranaphthylene | C8C6C6C6C6C6C6C6C6 |
| nonacene | C6C6C6C6C6C6C6C6C6 |
| nonaphene | C6C6C6C6C6C6C6C6C6 |
| nonahelicene | C6C6C6C6C6C6C6C6C6 |
| octacene | C6C6C6C6C6C6C6C6 |
| octaphene | C6C6C6C6C6C6C6C6 |
| pyranthrene | C6C6C6C6C6C6C6C6 |
| octahelicene | C6C6C6-C6C6C6C6C6 |
| hexaphenylene | C12C6C6C6C6C6C6 |
| heptacene | C6C6C6C6C6C6C6 |
| heptaphene | C6C6C6C6C6C6C6 |
| trinaphthylene | C6C6C6C6C6C6C6 |
| coronene | C6C6C6C6C6C6C6 |
| heptahelicene | C6C6C6C6C6C6C6 |
| rubicene | C6C6C6C6C6C5C5 |
| hexacene | C6C6C6C6C6C6 |
| hexaphene | C6C6C6C6C6C6 |
| hexahelicene | C6C6C6C6C6C6 |
| tetraphenylene | C8C6C6C6C6 |
| pentacene | C6C6C6C6C6 |
| pentaphene | C6C6C6C6C6 |
| perylene | C6C6C6C6C6 |
| picene | C6C6C6C6C6 |
| pleiadene | C7C6C6C6 |
| tetracene | C6C6C6C6 |
| tetraphene | C6C6C6C6 |
| chrysene | C6C6C6C6 |
| pyrene | C6C6C6C6 |
| triphenylene | C6C6C6C6 |
| aceanthrylene | C6C6C6C5 |
| acephenanthrylene | C6C6C6C5 |
| fluoranthene | C6C6C6C5 |
| anthracene | C6C6C6 |
| phenanthrene | C6C6C6 |
| phenalene | C6C6C6 |
| fluorene | C6C6C5 |
| acenaphthylene | C6C6C5 |
| biphenylene | C6C6C4 |
| s-indacene | C6C5C5 |
| as-indacene | C6C5C5 |
| heptalene | C7C7 |
| azulene | C7C5 |
| naphthalene | C6C6 |
| indene | C6C5 |
| pentalene | C5C5 |
P-26.0 IntroductionP-26.0 INTRODUCTION
P-26.1 Concepts and terminology
P-26.2 Components of phane parent names
P-26.3 Superatom locants and amplificant attachment locants
P-26.4 Numbering of phane parent hydrides
P-26.5 Skeletal replacement (‘a’) nomenclature in phane nomenclature
P-26.6 Other aspects of phane nomenclature
This section is based on the publication ‘Phane nomenclature, Part I. Phane parent names’ (ref. 5) and this new edition contains no modifications or changes to the recommendations contained therein except a change in the usage of brackets for heterocyclic amplificants which require locants. When a phane system contains a heterocyclic amplificant with locants, they are enclosed in brackets (see P-26.4.2.2, example 1 and P-26.5.1, example 2).
Phane nomenclature is specific to cyclic or acyclic compounds composed of rings or ring systems directly linked to each other or linked by atoms or chains.
Cyclophanes are recognized as a class of compounds (refs. 19, 23). The term originally applied to compounds having two 1,4-phenylene groups held face to face by –[CH2]n– bridges (ref. 23). It now designates compounds having:
(a) saturated and/or mancude rings or ring systems, or assemblies of saturated and/or mancude rings or ring systems and;Phane nomenclature is used to name cyclophanes and has been extended to linear compounds (for the use of phane nomenclature in the generation of preferred IUPAC names see P-52.2.5).(b) atoms and/or saturated or unsaturated chains as alternate components of a large ring.
P-26.1 CONCEPTS AND TERMINOLOGY
Definitions of terms that will be encountered in the construction of phane names are given below. These terms refer to types of operations, to the components of phane names, and to details of structures involved in the operations.
P-26.1.1 Simplification and amplification
The fundamental operations of phane nomenclature are illustrated in Fig. 2.1. The operation proceeding from left to right is called simplification; the reverse operation is called amplification, or phane replacement.
The simplification operation illustrates the initial step in the process of constructing a phane name, namely, the replacement of nomenclaturally significant segments of a complex cyclic structure by single atom symbols, called superatoms, thus producing a simplified skeleton that can more easily be named. The phane parent hydride name is then formed from the name of the simplified skeleton and those of the cyclic components (called amplificants) that were simplified to superatoms. In contrast to other bonds associated with the amplificant, the bonds marked by arrows in Fig. 2.1 do not disappear in the simplification or amplification operations.
Fig. 2.1. Phane nomenclature conversion diagram
Graph B in Fig. 2.1 at which simplication ends and amplification starts is called the simplified skeleton of the phane parent hydride, or simply the simplified skeleton, and is represented by a simplified phane parent graph. Its name is the simplified skeletal name. A simplified skeletal name implies a specific skeletal numbering; its locants are the skeletal locants, which become the primary locants for the phane parent hydride. In Fig. 2.1, the skeletal locants are denoted by large numbers; they are the same in the simplified skeleton and in the phane parent skeleton.
P-26.1.3 Superatom and superatom locants
The ‘atoms’ of the simplified skeleton shown by the symbol • in positions ‘1’ and ‘4’ in graph B in Fig. 2.1 that appear on simplification and disappear in amplification are called superatoms. Their locants are called superatom locants.
P-26.1.4 Amplificant, amplification prefix, and amplificant locants.
A multiatomic unit (a ring or a ring system) of structure replacing a superatom in the amplification operation is called an amplificant; the six-membered rings in Graph A are amplificants. They are expressed in a phane parent name by amplification prefixes. Each such prefix implies a specific numbering of the amplificant; the respective locants are called amplificant locants and are shown as the smaller numbers in Graph A.
P-26.1.5 Attachment atoms and attachment locants.
The atoms of an amplificant to which the bonds marked by arrows in Fig. 2.1 are attached are called attachment atoms and their locants are attachment locants. In Graph A in Fig. 2.1, amplificant locants ‘1’ and ‘4’ are the attachment locants of the upper ring and amplificant locants ‘1’ and ‘3’ are the attachment locants of the lower ring.
P-26.1.6 Phane parent skeleton, phane parent name, and phane parent hydrides
The skeletal graph at the start of the simplification operation or resulting from an amplification operation is called a phane parent skeleton. Correspondingly, the combination of the simplified skeletal name, amplification prefixes, and the appropriate superatom and attachment locants, is called a phane parent name. The term parent implies that it can be combined with names for other components derived from the operations of systematic nomenclature of organic chemistry, such as substituent prefixes, hydrogenation prefixes and endings, and characteristic group suffixes. In the absence of such other components, the compound is a phane parent hydride, which means that the name implies the order (valence) of all bonds of the skeletal parent and thus the number of hydrogen atoms attached to each of the skeletal atoms.
P-26.2 COMPONENTS OF PHANE PARENT NAMES
P-26.2.1 Simplified skeletal namesP-26.2.1 Simplified skeletal names
P-26.2.2 Amplification prefixes
P-26.2.3 Multiple identical amplificants
A simplified skeletal name consists of the term ‘phane’ preceded by a prefix denoting the structure of the simplified skeleton; this name is a parent for amplification but for no other operation. The simplification operation must be done in such a way that the amplificants can be expressed by amplification prefixes (see P-26.2.2).
A bond order of one is assumed for all bonds expressed by a simplified skeletal name. Atoms not identified by superatoms represent, by convention, carbon atoms with a bonding number (valence) of four in accordance with the principles of nomenclature of organic compounds.
Superatoms of a simplified skeletal name are assigned the lowest locants or the lowest set of locants, consistent with the numbering of the skeletal class to which it belongs. The lowest set of locants is the one that has the locant with the lowest numerical value at the first point of difference, when the sets are compared term by term in order of increasing value (see P-14.3.5).
Four types of simplified skeletal structures are described below, unbranched acyclic (P-26.2.1.1), monocyclic (P-26.2.1.2), polycyclic von Baeyer (P-26.2.1.3), and spiro (P-26.2.1.4).
Names of simplified skeletons consist of, in order, a prefix (‘cyclo’, ‘bicyclo’, ‘spiro’, etc.) indicating the type of structure, a numerical term ‘di’, ‘tri’, ‘tetra’, etc. indicating the number of nodes (including those designating superatoms), and the term phane. No prefix is used to name linear phanes. The nodes are numbered in accordance with the recommended numbering for each type of structure, as indicated in Chapter P-2. Superatoms are given the lowest possible locants.
P-26.2.1.1 Unbranched acyclic simplified skeletal structures
Example:
Example:
Example:
Example:
P-26.2.2.1 Naming amplification prefixes
Names of amplificant prefixes are those of allowed rings or ring systems (see P-26.2.2.2.1) modified by changing the final letter ‘e’ to ‘a’, or adding the letter ‘a’ when no final letter ‘e’ is present.
Examples:
| pyrrole (PIN) | pyrrola (preferred prefix) |
| furan (PIN) | furana (preferred prefix) |
| pyran (PIN) | pyrana (preferred prefix) |
| naphthalene (PIN) | naphthalena (preferred prefix) |
| anthracene (PIN) | anthracena (preferred prefix) |
P-26.2.2.2 Parent hydride names for deriving amplification prefixes
P-26.2.2.2.1 Allowed parent hydrides
An amplification prefix can be derived from monocyclic rings and polycyclic ring systems having the maximum number of noncumulative double bonds (mancude), bridged fused ring systems, saturated monocyclic rings, saturated bicycloalkanes and polycycloalkanes (von Baeyer hydrocarbons), and spiro alkanes. In addition stereoparents are allowed, such as ‘gonane’ or ‘morphinan’ (see Chapter P-10). Numbering of the parent is retained.
P-26.2.2.2.2 Disallowed names of parent hydrides
(a) the following parent hydride names are not allowed:
(1) spirobi names such as ‘1,1′-spirobi[indene]’ (PIN);(b) modified parent hydride names (the corresponding modifications are made once the phane parent hydride has been fully constructed):(2) spiro ring systems with at least one fused ring system or polycycloalkane ring system, such as ‘spiro[[1,3]dioxolane-2,1′-indene]’ (PIN) and ‘spiro[bicyclo[2.2.2]octane-2,1′-cyclohexane]’ (PIN)
(3) ring assembly names, such as ‘1,1′-biphenyl’ (PIN).
(1) by ’hydro’ prefixes, such as ‘9,10-dihydroanthracene’ (PIN);(c) functional parent structures having retained names, such as ‘benzoic acid’ (PIN) and ‘aniline’ (PIN).(2) by ‘-ene’ or ‘-yne’ endings, such as ‘cyclohexene’ (PIN);
(3) by skeletal replacement (‘a’) terms, such as ‘1-azabicyclo[3.2.1]octane’ (PIN);
(4) by suffixes, such as ‘cyclohexanecarboxylic acid’ (PIN) and ‘cyclohexanone’ (PIN);
(5) by substitutive prefixes, such as ‘ethylbenzene’ (PIN).
(d) names of cyclic compounds formed by functional class nomenclature, such as ‘benzyl chloride’.
(e) partially hydrogenated parent hydride names having retained names, such as ‘indane’ and ‘chromane’.
P-26.2.2.3 Order of citation of amplification prefixes
Amplification prefixes are cited in a name in decreasing order of their ring seniority (see P-44.2).
P-26.2.3 Multiple identical amplificants
Amplificants occurring more than once in a parent phane skeleton are expressed by use of an appropriate multiplicative term, either ‘di’, ‘tri’, etc. or ‘bis’, ‘tris’, etc. It is not necessary that the identical amplification prefixes have also identical locants.
P-26.2.3.1 The multiplying prefixes ‘di’, ‘tri’, ‘tetra’, etc. are used in front of simple amplification prefixes, for example dibenzena, tripyridina.
P-26.2.3.2 The multiplying prefixes ‘bis’, ‘tris’, ‘tetrakis’, etc. are used before an amplification prefix when it begins with a multiplying prefix as in ‘bicyclo[2.2.1]heptane’ (PIN), ‘1,3-dioxole’ (PIN); or it begins with a name component that could be preceded by a multiplying prefix indicating a multiple occurrence of that name component, as in ‘1,4-oxazine’ (PIN), ‘2-benzoxepine’ (PIN), and ‘1,4- methanonaphthalene’ (PIN).
P-26.3 SUPERATOM LOCANTS AND AMPLIFICANT ATTACHMENT LOCANTS
After the simplified skeletal name and the amplification prefix names have been determined, the phane parent hydride name is completed by adding the locants for the superatoms and the attachment locants. These locants are cited before an amplification prefix; the superatom locant is cited first followed by the attachment locants enclosed in parentheses.
P-26.3.1 Superatom locants
Superatom locants are assigned the lowest locants of the simplified skeleton consistent with the numbering of the class to which it belongs. An amplification prefix preceded by a multiplicative term indicating the presence of like amplificants requires the appropriate number of superatom locants, which are cited in ascending numerical order.
When like amplificants also have identical attachment locants, their attachment locants are arranged in ascending numerical order of the first cited superatom locant.
P-26.3.2 Attachment locants
The locants in parentheses in a phane parent hydride name are the attachment locants of the amplificant whose position in the phane parent skeleton is specified by the preceding superatom locant. The specific order of the attachment locants in the set describes precisely how their respective amplificant is attached to the rest of the phane parent skeleton. Amplificants retain the locants of the cyclic parent hydride from which they are derived.
P-26.3.2.1 Identical attachment locant sets for multiple identical amplificants are cited only once in a name; they follow the set of superatom locants corresponding to the identical amplificants.
Example:
Example:
The following rules are used for numbering phane parent hydrides. These rules are hierarchical; that is, each particular rule is applied only to alternatives not eliminated by preceding rules.
P-26.4.1 Numbering of phane parent skeletons and amplificants
P-26.4.1.1 The numbering of a phane parent skeleton is first determined by the rules governing the appropriate skeletal class to which it belongs. When because of skeletal symmetry, these rules leave alternatives, the numbering that gives the lowest set of locants for the superatoms is selected. The lowest set of locants is the one that has the lowest numerical value at the first point of difference, when the sets are compared term by term in increasing numerical value, as defined in P-14.3.5.
P-26.4.1.2 Numbering of an amplificant is determined primarily by the numbering rules that apply to the parent name from which the amplification prefix is derived. When there is a choice, the general rule of lowest locants is used, as described in the preceding rule.
These two rules, P-26.4.1.1 and P-26.4.1.2, are illustrated by the following examples.
Examples:
3(5,2)-pyridina-1(3,1)-piperidina-6(3,1)-naphthalenacyclononaphane (PIN)
[not 1(5,2)-pyridina-3(3,1)-piperidina-7(3,1)-naphthalenacyclononaphane
nor 7(5,2)-pyridina-5(1,3)-piperidina-1(3,1)-naphthalenacyclononaphane;
the superatom locant set of the PIN, ‘1,3,6’, is lower than the locant set ‘1,3,7’ or ‘1,5,7’
in either of the other names. see P-26.4.1.1]
[not 3(2,5)-pyridina-1(1,3)-piperidina-6(1,3)-naphthalenacyclononaphane;
the first locant of each the attachment locant sets ‘(2,5)’, ‘(1,3)’, and ‘(1,3)’
for the pyridine, piperidine, and naphthalene amplificants, respectively,
is not the locant adjacent to the lower locant of the simplified parent skeleton, see P-26.3.2.2]
Examples:
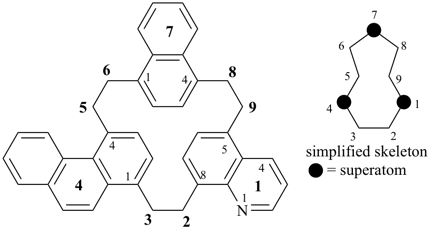
3(2,5)-pyridina-1,7(1),5(1,4)-tribenzenaheptaphane (PIN)
[not 5(5,2)-pyridina-1,7(1),3(1,4)-tribenzenaheptaphane;
the locant ‘3’ for the pyridine amplificant in the PIN is lower than ‘5’, see P-26.3.1]
Examples:
1,4(1,4)-dibenzenacyclohexaphane (PIN)
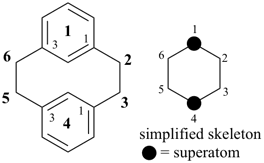
1,4(1,3)-dibenzenacyclohexaphane (PIN)
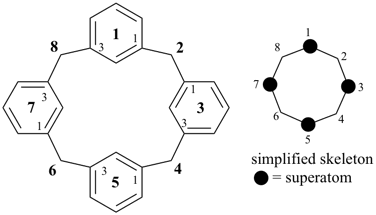
1,3,5,7(1,3)-tetrabenzenacyclooctaphane (PIN)
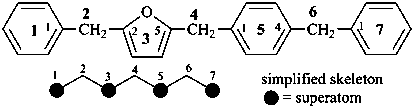
3(2,5)-furana-1,7(1),5(1,4)-tribenzenaheptaphane (PIN)
[not 3(5,2)-furana-1,7(1),5(1,4)-tribenzenaheptaphane;
for superatom 3 the lower attachment locant is not adjacent
to the lower-numbered atom 2 of the simplified skeleton]
Numbering alternatives are found in symmetrical simplified skeletons when amplification by a single unsymmetrical amplificant or by identical amplificants having different attachment locants removes the symmetry. Choice among such alternatives is made according to the following rules.
P-26.4.2.1 When a single amplificant is unsymmetrical, the lower locant of the phane parent skeleton must be adjacent to the lower attachment locant of the amplificant.
Examples:
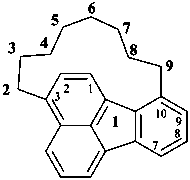 | not | 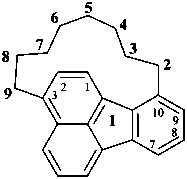 |
| (I) | (II) | |
 1(3,10)-fluoranthenacyclononaphane (I) [not 1(10,3)-fluoranthenacyclononaphane (II); the lower amplificant locant ‘3’ must be adjacent to the lower simplified parent skeletal locant ‘2’] 3,10-octanofluoranthene (PIN) [see P-52.2.5.1 (1)] | ||
P-26.4.2.2 When two amplificants can be given the lower of two superatom locants, the lower locant is assigned to the superatom representing the amplificant with the lower set of attachment locants. When necessary, this procedure is applied to other amplificants in accordance with their order of seniority until two or more identical amplificants have different attachment locants (see last example).
Examples:
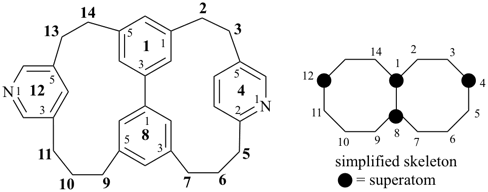
4(5,2),12(3,5)-dipyridina-1,8(1,3,5)-dibenzenabicyclo[6.6.0]tetradecaphane (PIN)
[not 4(3,5),12(2,5)-dipyridina-1,8(1,3,5)dibenzenabicyclo[6.6.0]tetradecaphane;
for the pyridine amplificants the attachment locant set ‘(5,2)’, when compared in ascending numerical order,
that is, ‘(2,5)’, is lower than ‘(3,5)’; therefore in the PIN,
the pyridine amplificant with the locant set ‘(5,2)’ must be associated with the lower superatom locant, ‘4’]
1(4,2), 4(5,2),7(2,6)-tripyridinacyclononaphane (PIN)
[the attachment locant sets ‘(2,4)’, ‘(2,5)’, and ‘(2,6)’ must be assigned to superatoms ‘1’, ‘4’, and ‘7’,
respectively; the arrangement of the locants in each set is governed by P-26.3.2.2]
3(2,5),5(2,6)-dipyridina-1,7(1)-dibenzenaheptaphane (PIN)
[not 3(6,2),5(5,2)-dipyridina-1,7(1)-dibenzenaheptaphane;
the attachment locants ‘2,5’ and ‘2,6’ of the pyridine amplificants
must be assigned to superatoms ‘3’ and ‘5’, respectively (see P-26.3.2)]
Examples:
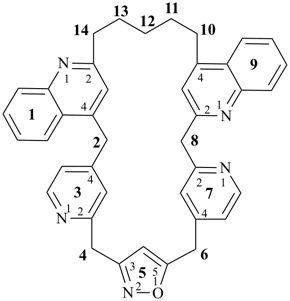 | not | 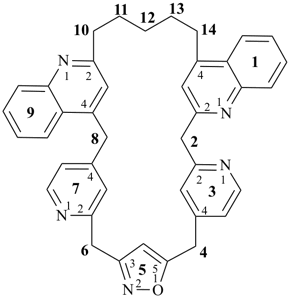 |
| (I) | (II) | |
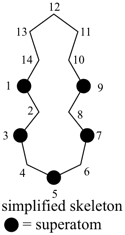 | 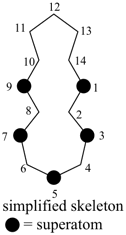 | |
| (I) | (II) | |
| 1(4,2),9(2,4)-diquinolina-3,7(4,2)-dipyridina-5(3,5)-[1,2]oxazolacyclotetradecaphane (I) (PIN) [not 1(2,4),9(4,2)-diquinolina-3,7(2,4)-dipyridina-5(5,3)-[1,2]oxazolacyclotetradecaphane (II); each of the identical pairs of amplificants, quinolina and pyridina, have identical attachment locants, ‘(2,4)’ and ‘(2,4)’, respectively; the single unsymmetrical amplificant, 1,2-oxazola, remains to which P-26.4.2.1 applies; its lower attachment locant ‘3’ must be adjacent to the lower locant of the simplified parent skeleton, ‘4’] | ||
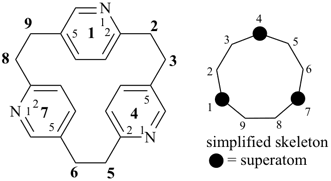
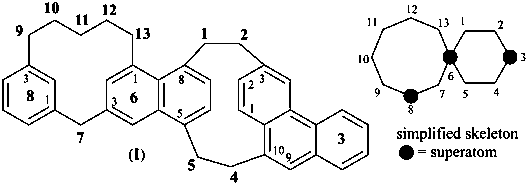
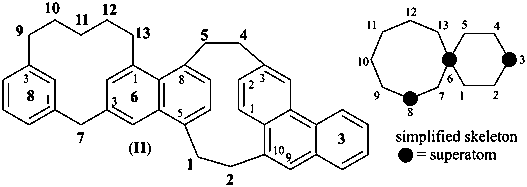
3(3,10)-phenanthrena-6(8,5,3,1)-naphthalena-8(1,3)-benzenaspiro[5.7]tridecaphane (I) (PIN)
[not 3(10,3)-phenanthrena-6(5,8,3,1)-naphthalena-8(1,3)-benzenaspiro[5.7]tridecaphane (II);
the set of attachment locants ‘(3,10)(8,5,3,1)(1,3)’ in the PIN cited for comparison
in the order of the increasing value of their corresponding superatom locants,
is lower than the corresponding set of attachment locants, ‘(10,3)(5,8,3,1)(1,3)’]
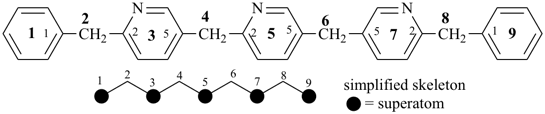
3,5(2,5),7(5,2)-tripyridina-1,9(1)-dibenzenanonaphane (PIN)
[not 3(2,5),5,7(5,2)-tripyridina-1,9(1)-dibenzenanonaphane;
the attachment locants set ‘(2,5)(2,5)(5,2)’ in the PIN, compared
in the order of the increasing value of their corresponding superatom locants,
is lower than the corresponding set of attachment locants,‘(2,5)(5,2)(5,2)’]
P-26.4.3.1 In a phane parent hydride, the locants for atoms that do not belong to amplificants are the locants of the simplified skeleton. However, locants for the atoms of the amplificants must be distinguished from the arabic number locants of the simplified skeleton. Thus, locants for amplificant atoms are constructed by citing the actual locants of the amplificant as superscripts to the locant of the superatom that represents the amplificant in the simplified skeleton.
P-26.4.3.2 In a substituted phane parent hydride name, a series of composite locants based on the superatom locant must not be contracted. As is the rule for citing locants in front of detachable prefixes, there must be a number of locants corresponding to the multiplying prefix, ‘di’, ‘tri’, etc. in front of the prefix.
P-26.4.3.3 The seniority of a composite locant is determined first on the basis of its primary locants, i.e., the locants of the phane parent skeleton, and, if these locants are identical, on the basis of the complete composite locant itself, i.e., the primary locant and its superscripts.
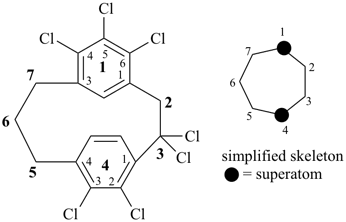
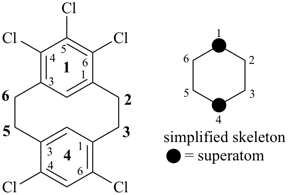
14,15,16,44,46-pentachloro-1,4(1,3)-dibenzenacyclohexaphane (PIN)
[not 14,16,44,45,46-pentachloro-1,4(1,3)-dibenzenacyclohexaphane;
the primary locant set ‘1,1,1,4,4’ is lower than the locant set ‘1,1,4,4,4’]
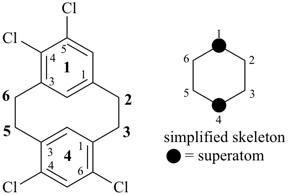
14,15,44,46-tetrachloro-1,4(1,3)-dibenzenacyclohexaphane (PIN)
[not 14,16,44,45-tetrachloro-1,4(1,3)-dibenzenacyclohexaphane;
the primary locant sets ‘1,1,4,4’ are identical
but the composite locant set in the PIN, ‘14,15,44,46’, is lower than ‘14,16,44,45’ ]
Example:
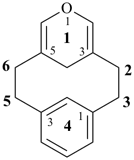
Skeletal replacement (‘a’) nomenclature is applied in two ways in phane nomenclature:
(1) to name phane parent hydrides having heteroatoms located in the simplified parent skeleton, i.e., heteroatoms not in names of amplification prefixes;The general principles, conventions, and rules described for skeletal replacement (‘a’) nomenclature in Section P-15.4 are fully applicable to the appropriate phane parent hydrides.(2) to indicate heteroatoms in heterocyclic amplificants whose names cannot be used as amplification prefixes because they, themselves, are named by skeletal replacement (‘a’) nomenclature, for example, heteromonocycles having more than ten members and polycyclic von Baeyer systems.
P-26.5.1 Skeletal replacement (‘a’) nomenclature in simplified phane names is accomplished in two steps. First, the parent phane hydrocarbon is named and then the heteroatoms are denoted by means of nondetachable ‘a’ prefixes cited in front of the name so created. Locants for the heteroatoms are assigned according to the numbering of the simplified parent skeleton.
Examples:
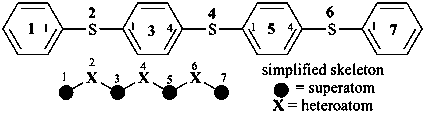
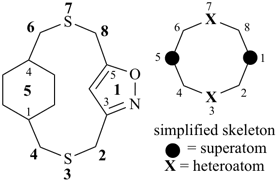
Step 1: 1(3,5)-[1,2]oxazola-5(1,4)-cyclohexanacyclooctaphane
Step 2: 3,7-dithia-1(3,5)-[1,2]oxazola-5(1,4)-cyclohexanacyclooctaphane (PIN)
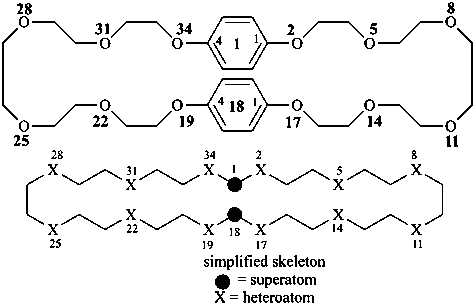
Locants for heteroatoms in amplificants are assigned according to the numbering of the simplified skeleton and the position of heteroatoms in the amplificants following the instructions in P-26.4 for substituent locants. Thus, positions of heteroatoms in amplificants are described by composite locants.
Example:
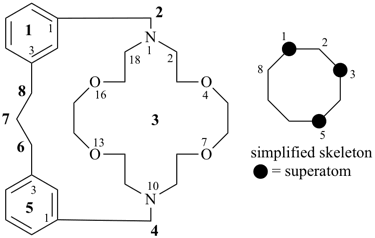
P-26.5.3 Simultaneous skeletal replacement (‘a’) in simplified skeletal names and amplificants
When skeletal replacement occurs in both simplified skeletons and in amplificants both P-26.5.1 and P-26.5.2 are applied.
Example:
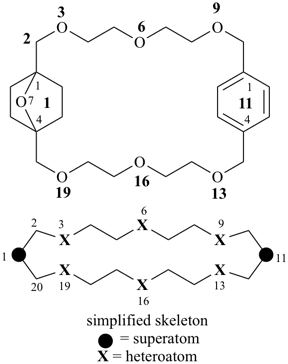
When there is a choice for numbering heterocyclic amplificants named by skeletal replacement (‘a’) nomenclature or for numbering simplified phane skeletons in which skeletal replacement has taken place, the following criteria are applied, in the order given, until a decision is reached.
P-26.5.4.1 Lowest locants are assigned to heteroatoms considered without regard to the nature of the heteroatom, first for the set of primary locants for the heteroatoms, i.e., the locants of the simplified skeleton (without including any superscript numbers), and then, if these locants are identical, with regard to the set of the complete heteroatom locants, which include the primary and the superscript numbers.
Examples:
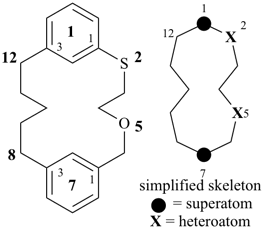 | not | 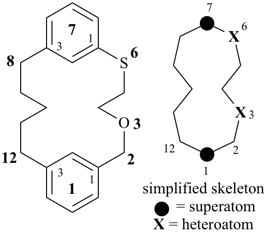 |
| Step 1 : 1,7(1,3)-dibenzenacyclododecaphane [(I) and (II)] Step 2: 5-oxa-2-thia-1,7(1,3)-dibenzenacyclododecaphane (I) (PIN) [not 3-oxa-6-thia-1,7(1,3)-dibenzenacyclododecaphane (II); the locant set of the heteroatoms in (I) , ‘2,5’, is lower than the locant set in (II), ‘3,6’] | ||
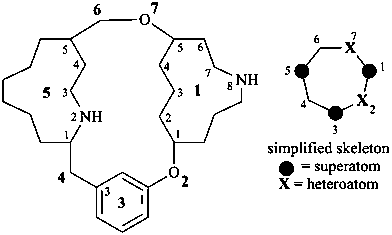
Step 1: 1,5(1,5)-dicycloundecana-3(1,3)-benzenacycloheptaphane
Step 2: 2,7-dioxa-18,52-diaza-1,5(1,5)-dicycloundecana-3(1,3)-benzenacycloheptaphane (PIN)
[not 4,6-dioxa-12,58-diaza-1,5(1,5)-dicycloundecana-3(1,3)-benzenacycloheptaphane;
the primary locant set for the heteroatoms in the PIN, cited for comparison
in ascending order ‘1,2,5,7’, is lower than the locant set ‘1,4,5,6’]
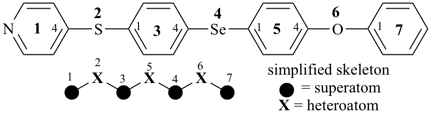
6-oxa-2-thia-4-selena-1(4)-pyridina-3,5(1,4),7(1)-tribenzenaheptaphane (PIN)
[not 2-oxa-6-thia-4-selena-7(4)-pyridina-1(1),3,5(1,4)-tribenzenaheptaphane;
the senior amplificant ‘pyridina’ must receive the lowest possible locant]
Examples:
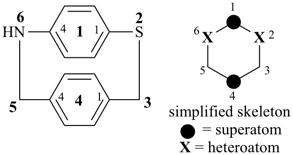
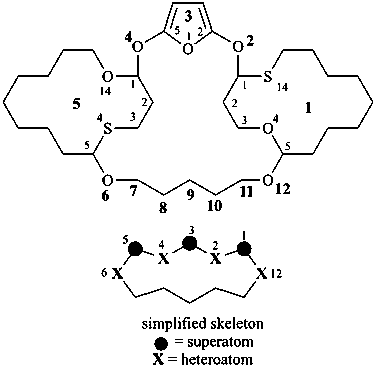 | not | 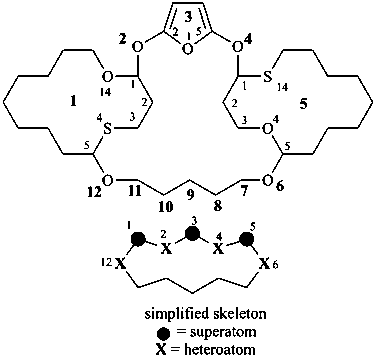 |
| (I) | (II) | |
| Step 1: 3(2,5)-furana-1,5(1,5)-dicyclotetradecanacyclododecaphane Step 2: 14,2,4,514,6,12-hexaoxa-114,54-dithia-3(2,5)-furana-1,5(1,5)-dicyclotetradecanacyclododecaphane (I) (PIN) [not 114,2,4,54,6,12-hexaoxa-14,514-dithia-3(2,5)-furana-1,5(1,5)-dicyclotetradecanacyclododecaphane (II); application of P-26.5.4.1 reveals that both the primary locant sets and the primary locant sets including the composite locants for the heteroatoms without regard to the kind of heteroatom are the same for both names, namely, ‘1,1,2,4,5,5,6,12’ and ‘14,114,2,4,54,514,6,12’, respectively. The primary locant sets for the senior prefix ‘oxa’ are also the same in both cases, ‘1,2,4,5,6,12’, but for the locant sets including composite locants, the locant set for the prefix ‘oxa’ in (I), the PIN, namely, ‘14,2,4,514,6,12’, is lower than the locant set, ‘114,2,4,54,6,12’, for oxa in (II)] | ||
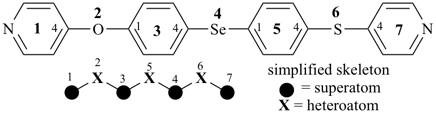
2-oxa-6-thia-4-selena-1,7(4)-dipyridina-3,5(1,4)-dibenzenaheptaphane (PIN)
[not 6-oxa-2-thia-4-selena-1,7(4)-dipyridina-3,5(1,4)-dibenzenaheptaphane;
the senior replacement (‘a’) prefix ‘oxa’ must receive the lowest locant]
Part II of phane nomenclature, ref. 6, discusses (1) phane parent hydrides substituted by characteristic groups cited as suffixes. Examples are given in P-62.2.5.3 for amines, in P-63.1.2 for hydroxy compounds, and in P-64.2.2.3 for ketones; and (2) the modification of the degree of hydrogenation; a topic more fully developed in Sections P-31.1.6 and P-31.2.3.3.4.
P-27.0 INTRODUCTION
This section is based on the publication ‘Nomenclature for the C60-Ih and C70-D5h(6) Fullerenes’ (ref. 10) and the new edition 2013 contains no modifications or changes to the recommendations therein.
A preliminary survey of the nomenclature and terminology of fullerenes was published in 1997 (ref. 25). This Section is based on the IUPAC Recommendations 2002 (refs. 10, 11), that deal in depth with two fullerenes, i.e. the most commonly known fullerene having 60 carbon atoms and one of its C70 homologues.
This Section is devoted to parent hydrides only (ref. 10). Derivatives are described in Chapter P-6, radicals and ions are discussed in Chapter P-7. Chapter P-9 includes a brief mention of fullerenes in the discussion on the configurational notation.
P-27.1 DefinitionsP-27.1 DEFINITIONS
P-27.2 Fullerene names
P-27.3 Numbering of fullerenes
P-27.4 Structurally modified fullerenes
P-27.5 Replacement of skeletal atoms
P-27.6 Addition of rings and ring systems to fullerenes
P-27.7 Other aspects of fullerene nomenclature
P-27.1.1 Fullerenes
Fullerenes are compounds composed solely of an even number of carbon atoms, which form a cage-like fused-ring polycyclic system with twelve five-membered rings and the rest six-membered rings (see ref. 10). The archetypal example is [60]fullerene, where the atoms and bonds delineate a truncated icosahedron. The term has been broadened to include any closed cage structure consisting entirely of three-coordinate carbon atoms.
(C70-D5h(6))[5,6]fullerene (PIN)
Fulleranes are fully saturated fullerenes, for example, C60H60.
P-27.1.3 Fulleroids
Heterofullerenes, norfullerenes, homofullerenes, and secofullerenes have been called ‘fulleroids’ (fullerene-like), because they resemble fullerenes in structure but do not conform to the definition of a fullerene as given above. It is convenient to refer to them as fulleroids and name them as modified fullerenes.
P-27.2 FULLERENE NAMES
P-27.2.1 Systematic names
The recommended systematic names for fullerenes include the number of carbon atoms, the point group symbol, the size of the rings, the relative arrangement of rings, and the term ‘fullerene’, all combined to give names, such as (C60-Ih)[5,6]fullerene (PIN) and (C70-D5h(6))[5,6]fullerene (PIN) for the two fullerenes discussed in this Section. The parenthetical prefix gives the carbon content and the point group symbol and the bracketed numbers indicate the ring sizes in the fullerene. The latter is important in fullerenes with rings other than five- and six-membered. The subscript (6) following the point group symbol D5h in the latter name indicates that the five-membered ring on the five-fold symmetry axis is surrounded by six-membered rings. This differentiates this fullerene from an isomeric fullerene which has five-membered rings surrounding the five-membered ring on the five-fold symmetry axis, which would have the name (C70-D5h(5))[5,6]fullerene (PIN).
The recommended names have the same information as the corresponding names used by the Chemical Abstracts Service (CAS), but in a different format. The corresponding CAS names are [5,6]fullerene-C60-Ih and [5,6]fullerene-C70-D5h(6), respectively (see ref. 10).
P-27.2.2 Trivial names
The names [60-Ih]fullerene and [70-Dh]fullerene (shortened to [60]fullerene and [70]fullerene in usage) given in the IUPAC Preliminary Survey (ref. 25) are names first introduced in the literature for the (C60-Ih)[5,6]... and (C70-D5h(6))[5,6]... fullerenes. They were based on a limited definition of fullerenes restricted to five- and six-membered rings. Since important information is missing from these names, they are considered as trivial names only for these specific compounds.
P-27.2.3 Preferred IUPAC names
IUPAC systematic names are preferred to CAS and trivial names. These names are not fully interchangeable. They each depend on a specific methodology for generating names of derivatives. But, most importantly, they do correspond to different numbering systems, one for IUPAC systematic and CAS names, another one for trivial names (see P-27.3). Preferred names for fullerenes and fullerene derivatives are those that use preferred components when a choice is possible (see P-52.2.6).
P-27.3 NUMBERING OF FULLERENES
Systematic numbering is not yet a fully solved issue in the nomenclature of fullerenes. The objectives are to achieve a continuous numbering and use a well defined starting point for all fullerenes. The criteria for numbering the (C60-Ih)[5,6]fullerene (PIN) and (C70-D5h(6))[5,6]fullerene (PIN) are discussed in the IUPAC publication (ref. 10). It is important to note that the systematic numbering used with IUPAC systematic names is derived from the system developed by the Chemical Abstracts Service and that the two systems are identical for these two specific fullerenes. The numbering associated with a trivial name may be different if it is based on principles such as ‘most reactive bond’. The two systems of numbering are shown below for three-dimensional structures and Schlegel representations.
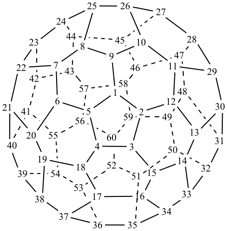 | 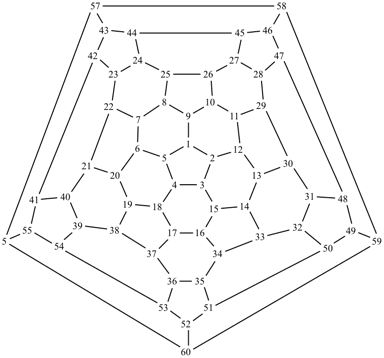 |
| 3-D Representation | Schlegel representation |
| (C60-Ih)[5,6]fullerene (PIN; systematic numbering) | |
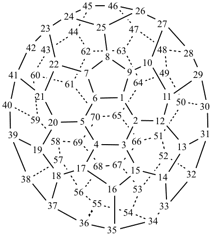 | 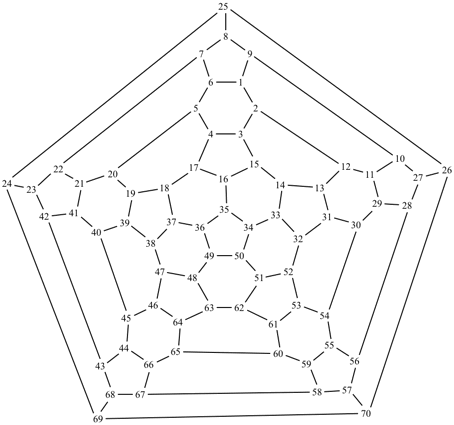 |
| 3-D Representation | Schlegel representation |
| (C70-D5h(6))[5,6]fullerene (PIN; systematic numbering) | |
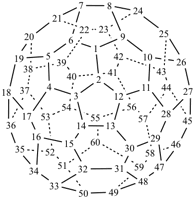 | 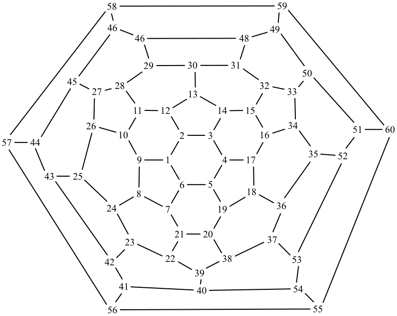 |
| 3-D Representation | Schlegel representation |
| (C60-Ih)fullerene (trivial numbering) | |
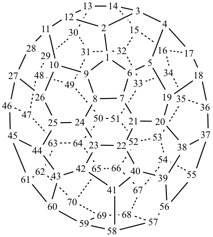 | 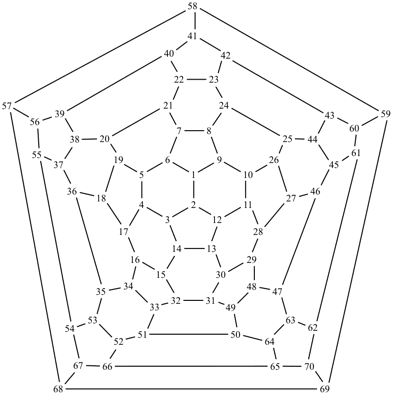 |
| 3-D Representation | Schlegel representation |
| [C70-D5h(6)]fullerene (trivial numbering) | |
Specific numberings of many other fullerene structures with rotational symmetry axes, including those with a discontiguous numbering pathway (according to systematic principles), and fullerenes with Cs and C1 point group symmetry have been published (ref. 11).
P-27.4 STRUCTURALLY MODIFIED FULLERENES
P-27.4.0 IntroductionP-27.4.0 Introduction
P-27.4.1 Homofullerenes
P-27.4.2 Norfullerenes
P-27.4.3 Secofullerenes
P-27.4.4 Cyclofullerenes
P-27.4.5 Combination of structure-modifying operations
The addition or removal of carbon atoms in a given fullerene does not create a new fullerene system described by a new number of carbon atoms and point group symbol, but is described by modifying the name of the unmodified fullerene using a nondetachable prefix, ‘homo’ or ‘nor’, respectively. In this way, fullerenes are parent structures analogous to fundamental structures used in the nomenclature of natural products (see Chapter P-10). Similarly, the cleavage of a bond or the formation of new bonds in specific situations is expressed by using the nondetachable prefixes ‘seco’ and ‘cyclo’, respectively, which are also used in the nomenclature of natural products. Contrary to the defined limited use of the prefixes ‘homo’, ‘nor’, ‘seco’, and ‘cyclo’ in the nomenclature of natural products, there is no such defined limitation in fullerene nomenclature.
P-27.4.1 Homofullerenes
The replacement of a carbon-carbon bond of a fullerene by a methylene (–CH2–) group is described by attaching the nondetachable prefix ‘homo’ to the name of the parent fullerene. The original numbering of the parent fullerene is retained. The location of the homo operation is described by a compound locant formed according to the method devised for insertion of a methylene group into a bond connector of a fundamental structure in the nomenclature of natural products (see P-101.3.2). The addition of two or more methylene groups is indicated by multiplicative prefixes ‘di’, ‘tri’, etc. placed at the front of the prefix ‘homo’. The compound locant is formed by adding the letter ‘a’ (‘b’, ‘c’, etc. if more than one methylene group replaces the bond) to the pair of locants which are the lowest locants consistent with the numbering of the fullerene, and enclosing the higher number in parentheses, for example ‘1(9)a’. Such compound locants must be used as locants in the names of the parent compounds, but, where there is no ambiguity, simple locants formed by adding the letter ‘a’ to the lowest locant, such as ‘1a’ for the case above, may be used in structural formulas and for citation of substituents. Locants to denote the addition of methylene group(s) are differently used in the nomenclature of natural products (see P-101.3.2). Locants used by CAS are also different and must not be used to describe the replacement by methylene groups in preferred IUPAC names of fullerenes (see Fu-4.1, ref. 10).
Example:
The nondetachable prefix ‘nor’ describes the deletion of one carbon atom from a fullerene structure; however, bonds attached to the atom removed are not reconnected as is the case in the nomenclature of natural products (see P-101.3.1.1). As a result, the connectivity of remaining atoms may be reduced from three to two, which requires the presence of hydrogen atoms. An even number of hydrogen atoms is implied in the name; if there is an odd number, one is expressed as indicated hydrogen because one carbon atom has changed from sp2 to sp3 hybridization. A connectivity of three may be satisfied by a heteroatom such as nitrogen or boron and a connectivity of two by a heteroatom such as oxygen or sulfur; these heteroatoms are introduced in a separate operation. Locants for the atoms must be as low as possible. The deletion of two or more carbon atoms is indicated by multiplicative prefixes ‘di’, ‘tri’, etc. placed at the front of the prefix ‘nor’.
It would not be helpful to set a precise number of carbon atoms that can be removed by the ‘nor’ operation for preferred IUPAC names; eventually, systematic ring nomenclature would provide a name which would be easier to understand than a polynorfullerene name. A realistic figure would depend much on whether blocks of carbon atoms or isolated carbon atoms are being removed. For the use of nor in the generation of preferred IUPAC fullerene names see P-52.2.6.1.
The use of the ‘nor’ prefix in the nomenclature of natural products is not the same and must not be used in, or adapted to, fullerene nomenclature.
Example:
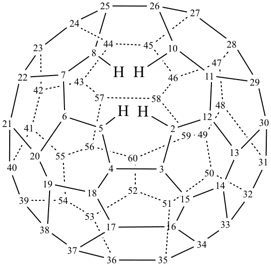
The nondetachable prefix ‘seco’ indicates the cleavage of one fullerene bond. Numbering of the parent fullerene is retained; where there is a choice, lowest possible locants are used to describe the seco operation. The valence requirements of the resulting carbon atoms with a connectivity of two are satisfied by hydrogen atoms following rearrangement of the double bonds. The hydrogen atoms are implied in the name of the secofullerene. The cleavage of two or more bonds is indicated by multiplicative prefixes ‘di’, ‘tri’, etc. placed at the front of the prefix ‘seco’.
It would not be helpful to set a precise number of bonds that can be cleaved by the ‘seco’ operation for preferred IUPAC names; eventually, systematic ring nomenclature would provide a name which would be easier to understand than a polysecofullerene name. A realistic figure would depend much on which bonds of the parent fullerene are being cleaved. For the generation of preferred IUPAC secofullerene names, see P-52.2.6.2.
The use of the ‘seco’ prefix in the nomenclature of natural products is not the same and must not be used in, or adapted to, fullerene nomenclature.
Example:
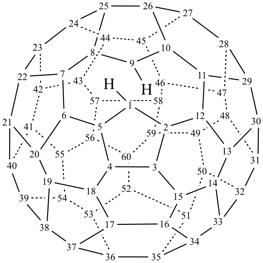
The nondetachable prefix ‘cyclo’ indicates the formation of a bond between two atoms of a modified fullerene or multifullerene structure. It almost always occurs in combination with one or more of the structure-modifying prefixes ‘homo’, ‘nor’, and ‘seco’. The formation of two or more bonds, when required, is indicated by multiplicative prefixes ‘di’, ‘tri’, etc. placed at the front of the prefix ‘cyclo’. No single fullerene is yet known that uses only ‘cyclo’. See the example under P-27.4.5.
P-27.4.5 Combination of structure-modifying operations
When more than one operation has been performed in a fullerene structure, the prefixes designating these operations are cited in names in the order ‘cyclo’, ‘seco’, ‘homo’, and ‘nor’ in front of the name of the fullerene. This is the reverse order that the operation indicated by the prefix has for assignment of lowest locants. ‘Nor’ prefixes are considered first for lowest locants and ‘homo’ prefixes are senior to ‘seco’ and ‘cyclo’ for lowest locants, since ‘homo’ locants may be needed for the latter operations. Locants for ‘cyclo’ and ‘seco’ prefixes are determined by the lowest set of locants, then by the order of citation of the locants in the name.
It would not be helpful to set a precise number of operations that can be performed on a parent fullerene structure for generation of preferred IUPAC names; eventually, systematic ring nomenclature would provide a name which would be easier to understand than a polysecofullerene name. A realistic number would depend much on the type of operations combined. For the use of structure-modifying prefixes in generating preferred IUPAC fullerene names, see P-52.2.6.
The rules for combinations of structure-modifying prefixes used in the nomenclature of natural products are not the same and must not be used in, or adapted to, fullerene nomenclature.
Example:
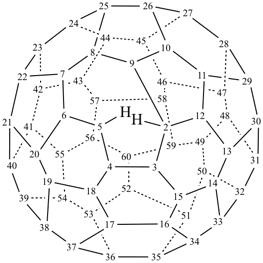
P-27.5.1 Fullerenes in which carbon atoms have been replaced by one or more heteroatoms are called ‘heterofullerenes’. Skeletal replacement (‘a’) nomenclature is used to name fullerenes in which carbon atoms have been replaced by heteroatoms having standard bonding numbers according to the ‘a’ prefixes of organic replacement nomenclature (see P-15.4) or bonding numbers indicated by the λ-convention (see P-14.1). The parent name is ‘fullerene’ if double bonds are present or possible in the parent fullerene; if double bonds are not possible, the parent name is ‘fullerane’. The heteroatoms include all elements capable of being tricoordinate, including metals and semimetals. Replacement names for fullerenes in which all carbon atoms have been replaced by the same or different heteroatoms are preselected names (see P-12.2). Replacement of carbon atoms by trivalent heteroatoms may result in the need for indicated hydrogen that receives lowest possible locants.
Examples:
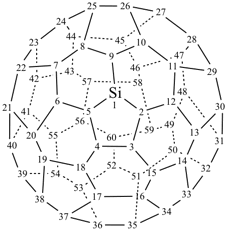
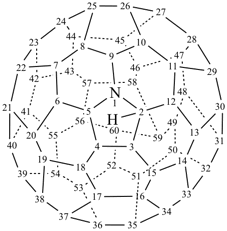
2H-1-aza(C60-Ih)[5,6]fullerene (PIN)
Example:
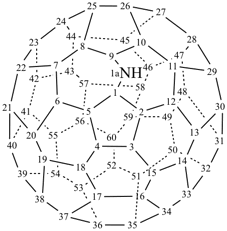
Addition of rings or ring systems to a fullerene is expressed as an ortho fusion operation, a bridging operation, or as a spirofusion operation as previously described in this Chapter.
P-27.6.1 Fullerenes and modified fullerenes ortho fused to organic rings or ring systemsP-27.6.1 Fullerenes and modified fullerenes ortho fused to organic rings or ring systems
P-27.6.2 Bridged fullerenes
P-27.6.3 Spiro fullerenes
Fullerenes or modified fullerenes that share an adjacent pair of atoms with an organic ring or ring system are named by adapting the principles of fusion nomenclature described in Section P-25. As in normal organic fused ring systems, the pair of atoms shared by the fullerene or modified fullerene and the organic ring or ring system is regarded as part of both components. However, unlike normal fused systems, each component retains its own bonding pattern and numbering. Because of the nature of bonding in fullerenes, the fusion bond is always a single bond and the fusion atoms cannot accept an ‘exo’ double bond. After fusion, nonfullerene components other than alicyclic bi- and polycyclic ring systems have the maximum number of noncumulative double bonds and indicated hydrogen is cited as needed.
Organic ring systems, including monocyclic rings and all polycyclic ring systems except spiro ring systems, are always cited as prefixes to the name of the fullerene or modified fullerene which is always the parent component. Each system retains its own name and numbering, both for indicating fusion sites and for indicating positions of substitution. The fullerene or modified fullerene locants are always unprimed, and primes are added to the fused organic ring or ring systems in the order described below. The fusion is described by citing the primed locants of the organic ring or ring system component and the unprimed locants of the fullerene or modified fullerene in that order, enclosed in brackets and separated by a colon. Locants for monocyclic hydrocarbons are omitted in preferred IUPAC names.
The methodology used to name fused derivatives of fullerenes is also used for naming fused fundamental structures in the nomenclature of natural products (see P-101.5). It is important to note that it must be integrally applied as described to generate IUPAC names. CAS names are different and more in line with the normal fusion operation in which the parent component is the senior ring or ring system. When this approach must be used, CAS names of fullerenes and modified fullerenes must be used. Furthermore, it is possible to replace fusion operations by bridging operations, as for example when the ring component is a cyclopropane or an oxirene ring. In these cases, bridging by using the bridging prefixes ‘methano’ or ‘epoxy’ (see P-25.4) can be an important alternative; this method was recommended in the preliminary survey (see ref. 25) and can be used in general nomenclature.
Examples:
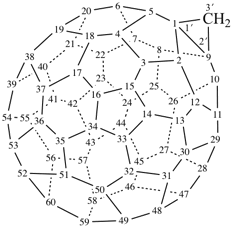
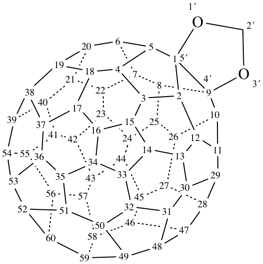
[1,3]dioxolo[4′,5′:1,9](C60-Ih)[5,6]fullerene (PIN)
1,9-(oxymethyleneoxy)(C60-Ih)[5,6]fullerene (see P-27.6.2)
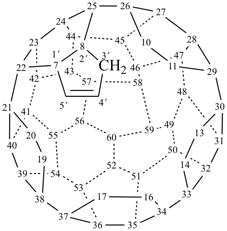
3′H-cyclopenta[7,8]-1,2,3,4,5,6,9,12,15,18-decanor(C60-Ih)[5,6]fullerene (PIN)
7,8-(prop-1-en-1,3-diyl)-1,2,3,4,5,6,9,12,15,18-decanor(C60-Ih)[5,6]fullerene
(see P-27.6.2)
Examples:
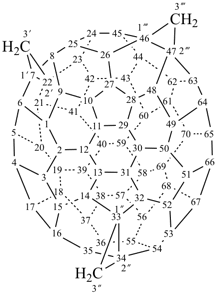
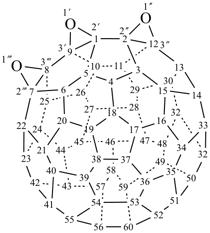
tris(oxireno)[2′,3′:1,9;2′′,3′′:2,12;2′′′,3′′′:7,8](C60-Ih)[5,6]fullerene (PIN)
1,9:2,12:7,8-tris(oxy)(C60-Ih)[5,6]fullerene (see P-27.6.2)
Bridges between nonadjacent atoms of a fullerene or modified fullerene are named and numbered according to established principles and rules for bridged fused ring systems (see P-25.4). Numbering of bridging atoms begins with the number following the highest number of the fullerene and starts with the atom adjacent to the fullerene atom with the higher locant number. Bridges between rings fused to fullerene and a parent fullerene, between two different rings fused to the same fullerene, or between two or more fullerenes joined by fused rings or ring systems are named using established bridge prefix names, but numbering begins with the bridge atom adjacent to the fused component with the least primed numbers and continues from this atom.
Examples:
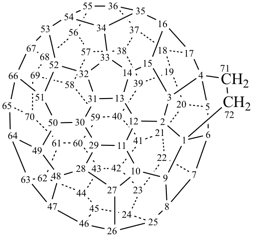
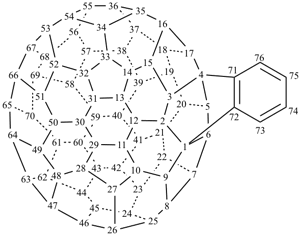
1,4-[1,2]benzeno(C70-D5h(6))[5,6]fullerene (PIN)
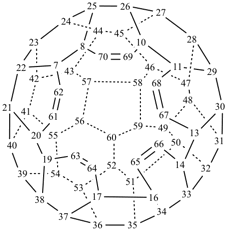
7,20:8,10:11,13:14,16:17,19-pentaetheno-1,2,3,4,5,6,9,12,15,18-decanor(C60-Ih)[5,6]fullerene (PIN)
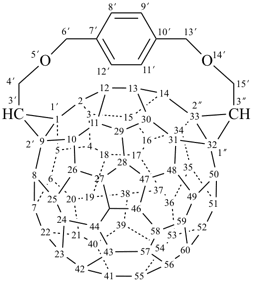
3′H,3′′H-3′,3′′-(methanooxymethano[1,4]benzenomethanooxymethano)dicyclopropa[1,9:32,33](C60-Ih)[5,6]fullerene (PIN)
1,9:32,33-[ethane-1,1,2-triyloxymethylene(1,4-phenylene)methyleneoxyethane-1,2,2-triyl](C60-Ih)[5,6]fullerene
Fullerenes cannot themselves form spiro compounds directly due to their specific connectivity and, as mentioned earlier, spiro ring systems are not fused to fullerenes. Spiro fullerenes formed from homofullerenes and fullerenes fused to organic ring systems follow the normal procedure for naming organic spiro systems that contain at least one polycyclic ring system, as described in P-24.5. Spiro fullerene parent hydrides will not necessarily have unprimed numbers as locants, as the alphanumerical order is used to name this type of spiro compound.
Example:
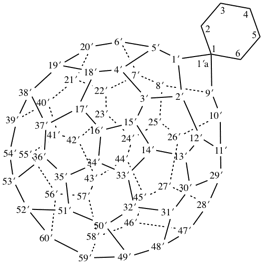
Part II of fullerene nomenclature, ref. 11, discusses rules for numbering a wide variety of fullerene structures:
(1) Fullerenes having at least one symmetry axis (Cn; n>1) and a contiguous helical numbering pathwayP-28 RING ASSEMBLIES(2) Fullerenes having at least one symmetry axis (Cn; n>1) but no contiguous helical numbering pathway
(3) Fullerenes belonging to the Cs point group and having a contiguous helical numbering pathway
(4) Fullerenes belonging to the Ci or C1 point groups and having a contiguous helical numbering pathway
P-28.0 IntroductionP-28.0 INTRODUCTION
P-28.1 Definitions
P-28.2 Ring assemblies of two identical cyclic systems
P-28.3 Unbranched ring assemblies of three through six identical cyclic systems
P-28.4 Ring assemblies of identical cyclic systems modified by skeletal replacement (‘a‘) nomenclature
P-28.5 Ring assembles of more than six identical cyclic systems
P-28.6 Branched ring assemblies of identical cyclic systems
P-28.7 Ring assemblies of nonidentical cyclic systems
Assemblies of cyclic parent hydrides linked by single or double bonds are described in this Section. They are named by using the so-called ‘Latin multiplying prefixes’, ‘bi’, ‘ter’, ‘quater’, etc., to indicate the number of rings or ring systems in the assembly.
Names of substituent groups described further in Section P-29 are used in this Section.
P-28.1 DEFINITIONS
Two or more cyclic systems (single rings or fused systems, alicyclic von Baeyer systems, spiro systems, phane systems, fullerenes) that are directly joined to each other by single or double bonds are called ‘ring assemblies’ when the number of such direct ring junctions is one less than the number of cyclic systems involved.
a fused ring system
two different ring systems
P-28.2.1 Ring assemblies with a single bond junction
Assemblies of two identical cyclic systems joined by a single bond are named by one of two methods:
(1) by placing the prefix ‘bi’ (see P-14.2.3) before the name of the corresponding parent hydride enclosed in parentheses, if necessary. Parentheses are used to avoid confusion with von Baeyer names;Each cyclic system is numbered in the traditional way, one with unprimed locants, the other with primed locants. Lowest possible locants must be used to denote the positions of attachment. These locants must be cited in preferred IUPAC names; they can be omitted in general nomenclature when no ambiguity would result.(2) by placing the prefix ‘bi’ (see P-14.2.3) before the name of the corresponding substituent group (for names of substituent groups, see P-29), enclosed in parentheses, if necessary.
The name biphenyl is retained as 1,1′-biphenyl.
Examples:
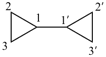
(2) 1,1′-biphenyl (PIN)
biphenyl
(1) 2,2′-bipyridine (PIN)
(2) 2,2′-bipyridyl
(1) 1,2′-binaphthalene (PIN)
(2) 1,2′-binaphthyl
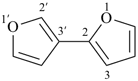
(1) 2,3′-bifuran (PIN)
(2) 2,3′-bifuryl
When two cyclic systems are linked by a double bond, method (2) described in P-28.2.1 is the only recommended method. Method (2) has also been used in which the presence of a double bond is indicated by the Greek letter Δ and the point of attachment of the ring is given by superscript locant numbers. This method is not continued in these recommendations; accordingly, ring assemblies of three or more identical cyclic systems interconnected by double bonds must be named by other methods (see P-31).
Examples:
2,2′-bi(bicyclo[2.2.1]heptanylidene) (PIN)
(not Δ2,2′-bicyclo[2.2.1]heptanylidene)
The citation of indicated hydrogen, if needed, at the front of the name of the ring assembly is a change from its position in previous editions (refs. 1 and 2) where it was kept with the name of the individual ring, for example, 2,2′-bi-2H-pyran.
Note: Citing the indicated hydrogen in front of the name of the ring assembly follows the method now adopted for bridged fused systems (see P-25.7.1.3.2) and spiro ring systems (see P-24.3.2). This method allows more assemblies of rings to be treated as ring assemblies, for example, 1H,2′H-2,4′-biindene. The advantages of this method become more obvious when naming derivatives requiring a divalent substituent group suffix, such as a ketone.
Examples:
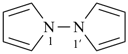
1,1′-bipyrrole (PIN)
(no indicated hydrogen needed)
(not 1,1′-bi-1H-pyrrole)
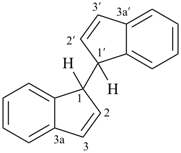
1H,1′H-1,1′-biindene (PIN)
(not 1,1′-bi-1H-indene)
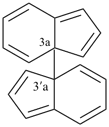
3a,3′a-biindene (PIN)
(no indicated hydrogen needed)
(not 3a,3′a-bi-3aH-indene)
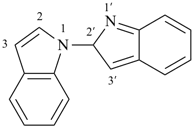
2′H-1,2′-biindole (PIN)
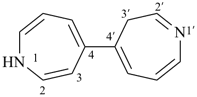
1H,3′H-4,4′-biazepine (PIN)
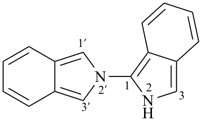
2H-1,2′-biisoindole (PIN)
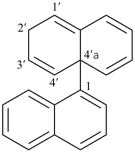
2′H-1,4′a-binaphthalene (PIN)
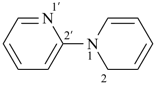
2H-1,2′-bipyridine (PIN)
2′H,3H-2,3′-bifuranylidene (PIN)
P-28.3.1 Unbranched ring assemblies consisting of three or more identical cyclic systems are named by placing the appropriate numerical Latin-based (see P-14.2.3) prefix, ‘ter’, ‘quater’, ‘quinque’, etc., before the name of the parent hydride corresponding to the repeating unit. Indicated hydrogen is applied as described above for two-component ring assemblies (see P-28.2.3).
Exceptionally, ring assemblies composed of three or more benzene rings are named by using the term ‘phenyl’. Each cyclic system of the assembly is numbered consecutively and each ring or ring system is numbered in its usual way provided the lowest locant set is selected and, if a choice remains, in the order of citation in the name. Composite locants (see P-14.3.1) are formed by citing the locants denoting positions in each ring or ring system as superscripts to the locants indicating the position of a cyclic system in the assembly (ref. 26). Locants indicating points of attachment are placed before the name of the assembly in ascending order; locants denoting junctions are separated by a comma and sets of junction locants are separated by a colon.
This is a new numbering system recommended for ring assemblies consisting of more than two rings or ring systems. The new system, which uses composite locants composed of primary locants and superscript numbers attached to them as described above, is recommended for preferred IUPAC names. The numbering method using serially primed locants used in earlier recommendations (refs. 1 and 2) may be used in general nomenclature.
Note: The elimination of primed locants for preferred IUPAC names is intended to improve the perception of the relationship between structures and names. The new numbering system for preferred IUPAC names is similar to that recommended in phane nomenclature (see P-26.1). For example, two alternative names are 1,1′:2′,1′′-tercyclopropane and 11,21:22,31-tercyclopropane, where the primary locants 1,2,3 correspond to the three rings of the assembly, and the superscript locants pertain to the locants of the points of attachment of the three rings.
Examples:
 | not |  |
| 11,21:22,31:33,41-quatercyclobutane (PIN) (not 11,21:23,31:32,41-quatercyclobutane; the locant set 11,21:22,31:33,41 is lower than the locant set 11,21:23,31:32,41) 1,1′:2′,1′′:3′′,1′′′-quatercyclobutane (not 1,1′:3′,1′′:2′′,1′′′-quatercyclobutane; the locant set 1,1′:2′,1′′:3′′,1′′′ is lower than 1,1′:3′,1′′:2′′,1′′′) | ||
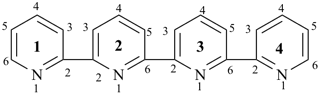
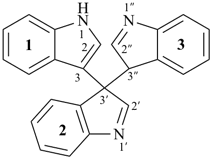
11H,33H-13,23:23,33-terindole (PIN)
1H,3′′H-3,3′:3′,3′′-terindole
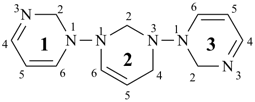
12H,22H,24H,32H-11,21:23,31-terpyrimidine (PIN)
2H,2′H,2′′H,4′H-1,1′:3′,1′′-terpyrimidine
Examples:

11,21:23,31:33,41-quaterphenyl (PIN)
1,1′:3′,1′′:3′′,1′′′-quaterphenyl
(not m-quaterphenyl)
P-28.4.1 Assemblies composed of identical heterocyclic compounds are named by using the names of parent hydrides, except in the case of heterocyclic compounds of the von Baeyer type and of monocyclic compounds having more than 10 members that are named by using skeletal replacement (‘a’) nomenclature. In the latter cases, the ‘a’ prefixes are placed at the front of the name of the hydrocarbon ring assembly.
Examples:
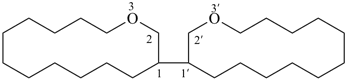
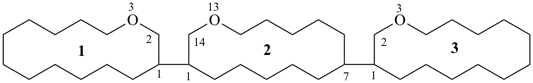
13,29,33-trioxa-11,21:27:31-tercyclotetradecane (PIN)
(not 13,213,33-trioxa-11,21:27:31-tercyclotetradecane; 13,29,33 is lower than 13,213,33)
3,3′′,9′-trioxa-1,1′:7′,1′′-tercyclotetradecane
(not 3,3′′,13′-trioxa-1,1′:7′,1′′-tercyclotetradecane; 3,3′′,9′ is lower than 3,3′′,13′)
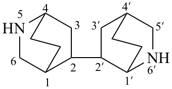
5,6′-diaza-2,2′-bi(bicyclo[2.2.2]octane) (PIN)
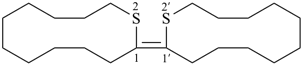
2,2′-dithia-1,1′-bi(cyclododecylidene) (PIN)
Examples:
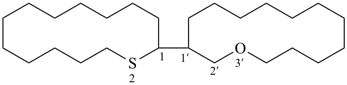
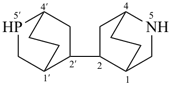
5-aza-5′-phospha-2,2′-bi(bicyclo[2.2.2]octane) (PIN)
[not 5′-aza-5-phospha-2,2′-bi(bicyclo[2.2.2]octane);
the locant 5 is lower than 5′, and the prefix ‘aza’ has priority to be assigned the lower locant]
Preferred IUPAC names for ring assemblies of more than six identical cyclic systems are generated using the principles of phane nomenclature (see P-26). Any of the methods discussed above (see P-28.2.1, P-28.3.1, P-28.3.2), as appropriate, can be used in general IUPAC nomenclature.
Examples:

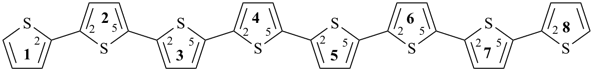
1,8(2),2,3,4,5,6,7(2,5)-octathiophenaoctaphane (PIN)
12,22:25,32:35,42:45,52:55,62:65,72:75,82-octithiophene
2,2′:5′,2′′:5′′,2′′′:5′′′,2′′′ ′:5′′′ ′,2′′′ ′′:5′′′ ′′,2′′′ ′′′:5′′′ ′′′,2′′′ ′′′ ′-octithiophene
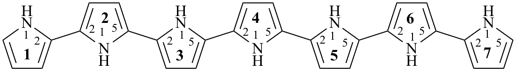
11H,21H,31H,41H,51H,61H,71H-1,7(2),2,3,4,5,6(2,5)-heptapyrrolaheptaphane (PIN)
11H,21H,31H,41H,51H,61H,71H-12,22:25,32:35,42:45,52:55,62:65,72-septipyrrole
1H,1′H,1′′H,1′′′H,1′′′ ′H,1′′′ ′′H,1′′′ ′′′H-2,2′:5′,2′′:5′′,2′′′:5′′′,2′′′ ′:5′′′ ′,2′′′ ′′:5′′′ ′′,2′′′ ′′′-septipyrrole
Preferred IUPAC names are formed by substituting into the longest unbranched assembly. The names of substituent groups are formed in accord with the methods described in P-29.3.5. If necessary, the criteria for selecting the principal chain are applied, namely, the longest chain, the lowest attachment locants in the order of citation, the maximum number of substituents, the lowest locants for substituents considered together, then alphanumerical order.
Examples:
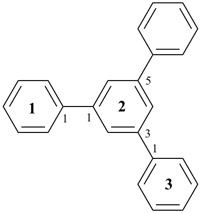
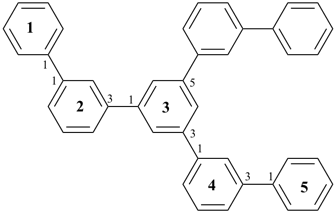
35-([1,1′-biphenyl]-3-yl)-11,21:23,31:33,41:43,51-quinquephenyl (PIN)
5′′-([1,1′-biphenyl]-3-yl)-1,1′:3′,1′′:3′′,1′′′:3′′′,1′′′ ′-quinquephenyl
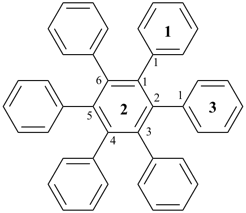
23,24,25,26-tetraphenyl-11,21:22,31-terphenyl (PIN)
(not 22,23,25,26-tetraphenyl-11,21:24,31-terphenyl
nor 22,24,25,26-tetraphenyl-11,21:23,31-terphenyl;
the locant set 11,21:22,31 is lower than either 11,21:24,31 or 11,21:23,31)
3′,4′,5′,6′-tetraphenyl-1,1′:2′,1′′-terphenyl
(not 2′,3′,5′,6′-tetraphenyl-1,1′:4′,1′′-terphenyl
nor 2′,4′,5′,6′-tetraphenyl-1,1′:3′,1′′-terphenyl;
the locant set 1,1′:2′,1′′ is lower than either 1,1′:4′,1′′ or 1,1′:3′,1′′)
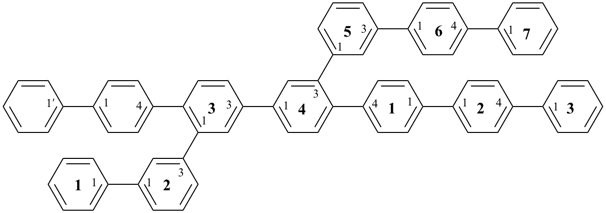
36-([1,1′-biphenyl]-4-yl)-44-([11,21:24,31-terphenyl]-14-yl)-1,7(1),2,3,4,5(1,3),6(1,4)-heptabenzenaheptaphane (PIN)
[not 32-([1,1′-biphenyl]-3-yl)-43-([11,21:24,31-terphenyl]-13-yl)-1,7(1),2,3,4,5,6(1,4)-heptabenzenaheptaphane;
the set of attachment locants (1,3)(1,3)(1,3)(1,3) for the superatom locants 2,3,4,5 is lower than (1,4)(1,4)(1,4)(1,4)]
Assemblies of cyclic systems that cannot be treated as ring assemblies as described above (P-28.2 and P-28.3) are simply assemblies of nonidentical cyclic systems and are named by substitutive nomenclature principles. Such cyclic hydrocarbon assemblies are discussed in Section P-61.2.2; and assemblies of nonidentical cyclic systems containing heteroatoms, such as Si, N, B, are considered in Section P-68. Phane nomenclature (see P-26), where applicable, is used to name ring assemblies composed of identical or nonidentical cyclic systems.
Ring assemblies of identical cyclic systems that are partially unsaturated or partially saturated can be modified either by the endings ‘ene’ or ‘yne’ (introduction of multiple bonds in saturated systems) or by using the prefixes ‘hydro/dehydro’ in mancude systems (see Section P-31). In some cases, especially in the case of benzene ring assemblies, assemblies of nonidentical cyclic systems result from such operations.
P-29 PREFIXES DENOTING SUBSTITUENT GROUPS DERIVED FROM PARENT HYDRIDES
P-29.0 IntroductionP-29.0 INTRODUCTION
P-29.1 Definitions
P-29.2 General methodology for naming substituent groups
P-29.3 Systematic names for simple substituent prefixes derived from saturated parent hydrides
P-29.4 Compound substituent groups
P-29.5 Complex substituent groups
P-29.6 Retained names for prefixes of simple substituent groups derived from the parent hydrides described in Chapter P-2
This Section includes the names of substituent groups derived from the parent hydrides described in Sections P-21 through P-28 and used as prefixes in substitutive nomenclature. The methodology for forming systematic prefixes is fully described in this Section; preferred IUPAC prefixes are identified, as well as preselected prefixes.
P-29.1 DEFINITIONS
Substituent groups derived from parent hydrides are used in many ways in the nomenclature of organic compounds and may be classified as simple substituent groups, compound substituent groups, and complex substituent groups.
The definitions for substituent groups derived from parent hydrides given below differ from those that were previously recommended (see A-2.3 in ref. 1 and R-4.1 in ref. 2).
P-29.1.1 A simple substituent group is an atom or group of atoms considered as a unit with one or more free valences. The methodology for naming such groups is described in P-29.2. The basic multiplicative prefixes ‘di’, ‘tri’, ‘tetra’, etc. denote the presence of more than one identical simple substituent group in a compound or complex substituent group, but see P-29.1.2 and P-29.1.3 for use of derived multiplicative prefixes, such as ‘bis’, ‘tris’, ‘tetrakis’.
P-29.1.2 A compound substituent group consists of a simple substituent group (the parent substituent group) to which is attached one or more simple substituent groups. Compound substituent group names are formed by combining the names of two or more simple substituent groups. There are three ways to accomplish this:
(1) by the substitutive operation;Compound substituent groups are formed by the substitution operation rather than by an additive operation unless the simple parent substituent group is not acceptable for substitution, i.e., it has no substitutable hydrogen atoms. Compound substituent groups formed by substitution are cited in names as prefixes, often called ‘substituent prefixes’. Compound substituent groups formed by addition are cited in names as prefixes, often called ‘concatenated prefixes’. The derived multiplicative prefixes ‘bis’, ‘tris’, ‘tetrakis’, etc. are used to multiply compound prefixes and to avoid ambiguity when the basic multiplying prefixes are already part of the name of a substituent group (see P-16.3).
(2) by an additive operation;
(3) by combined substitutive and additive operations.
Examples:
–CO-Cl
carbonochloridoyl (preferred prefix)
(a concatenated prefix; see P-13.3.4)
Example:
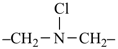
Examples:
>N-CH2-O-CH2-N<
oxybis(methylenenitrilo) (preferred prefix; a complex concatenated prefix)
Example:
The presence of free valences formally derived from the loss of one or more hydrogen atoms from a parent hydride is denoted by suffixes ‘yl’, ‘ylidene’, and ‘ylidyne’, together with multiplying prefixes indicating the number of free valences; lowest locants are assigned to all free valences as a set, then in the order ‘yl’, ‘ylidene’, ‘ylidyne’. In names, the suffixes are cited in the order ‘yl’, ‘ylidene’, ‘ylidyne’. The suffixes ‘ylidene’ and ‘ylidyne’ are used only to indicate the attachment of a substituent to a parent hydride or parent substituent by a double or triple bond, respectively.
| Monovalent | Divalent | Trivalent | Tetravalent, etc. |
| -yl | -diyl | -triyl | -tetrayl, etc. |
| -ylidene | -ylidyne | -ylylidyne, etc. | |
| -ylylidene | -diylidene, etc. | ||
| -diylylidene , etc. | |||
(1) The suffixes ‘yl’, ‘ylidene’, and ‘ylidyne’ replace the ending ‘ane’ of the parent hydride name. The atom with the free valence terminates a chain and always has the locant ‘1’, which is omitted from the name. This method is recommended primarily for saturated acyclic and monocyclic hydrocarbon substituent groups and for the mononuclear hydrides of silicon, germanium, tin, and lead. Substituent groups formed by this method are referred to as ‘alkyl-type substituent groups’;(2) The suffixes ‘yl’, ‘ylidene’, and ‘ylidyne’ are added to the name of the parent hydride with elision of the terminal letter ‘e’, if present, when followed immediately by the letter ‘y’. The locants for the atoms of free valences are as low as is consistent with any established numbering of the parent hydride and, except for mononuclear parent hydrides or the suffix ‘ylidyne’, the locant ‘1’ must be cited. This method is used to generate names of ‘alkanyl-type substituent groups’ that are simple substituent groups with free valences at positions other than ‘1’.
| Method (1) is no longer applicable to boron prefixes. |
For the application of these methods in generating preferred IUPAC names, see P-57.1.1.1.
Examples:
SiH3–
silyl (preselected prefix)
CH2=
methylidene (preferred prefix)
methanylidene
CH3 C≡
ethylidyne (preferred prefix)
ethanylidyne
trisilan-1-yl (preselected prefix)
propan-2-yl (preferred prefix)
1-methylethyl
C6H11–
cyclohexyl (preferred prefix)
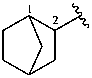
bicyclo[2.2.1]heptan-2-yl (preferred prefix)
bicyclo[2.2.1]hept-2-yl
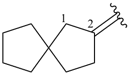
spiro[4.4]nonan-2-ylidene (preferred prefix)
spiro[4.4]non-2-ylidene
P-29.3.1 Substituent prefixes derived from mononuclear parent hydridesP-29.3.1 Substituent prefixes derived from mononuclear parent hydrides
P-29.3.2 Substituent prefixes derived from acyclic parent hydrides
P-29.3.3 Substituent prefixes derived from saturated cyclic parent hydrides
P-29.3.4 Substituent prefixes derived from mancude parent hydrides
P-29.3.5 Substituent prefixes derived from ring assemblies
P-29.3.6 Substituent prefixes derived from phane systems
Simple substituent groups derived from the mononuclear parent hydrides of carbon, silicon, germanium, tin, and lead (boron is no longer included) traditionally have been named by method (1) in P-29.2. Other mononuclear parent hydrides, i.e., those derived from O, F, Cl, Br, I, S, Se, Te, N, P, As, Sb, Bi, B, Al, Ga, In, and Tl, are named by method (2) in P-29.2. The prefix ‘amino’ is retained as the preselected prefix for –NH2, and the prefixes phosphino, arsino, and stibino may be used in general nomenclature. Substituent groups derived from mononuclear parent hydrides modified by the λ-convention are discussed in P-68.3.2 for elements of Group 15, in P-68.4.3 for elements of Group 16, and in P-68.5.1 for elements of Group 17.
| Prefixes derived from borane are now named only by method (2) in P-29.2, for example ‘boranyl’, ‘boranylidene’, and ‘boranylidyne’. |
Examples:
GeH3–
germyl (preselected prefix)
BH2–
boranyl (preselected prefix)
(not boryl)
CH2=
methylidene (preferred prefix)
methanylidene [see P-29.2 (2)]
SnH2=
stannylidene (preselected prefix)
BH=
boranylidene (preselected prefix)
(not borylidene)
HS–
sulfanyl (preselected prefix)
(no longer mercapto)
H2P–
phosphanyl (preselected prefix)
phosphino
HAs=
arsanylidene (preselected prefix)
(not arsinidine)
H2Al–
alumanyl (preselected prefix)
HAl=
alumanylidene (preselected prefix)
S=
sulfanylidene (preselected prefix)
thioxo
HSi≡
silylidyne (preselected prefix)
P≡
phosphanylidyne (preselected prefix)
(not phosphinidyne)
Names for simple substituent groups derived from names of acyclic parent hydrides are formed by both methods described in P-29.2.
P-29.3.2.1 Names for simple substituent groups derived from acyclic hydrocarbons with a single free valence at the end of the longest chain are traditionally formed according to method (1) of P-29.2. They are generically called ‘alkyl’, ‘alkylidene’, and ‘alkylidyne’ substituent groups. The free valences are assigned the locant 1, which is omitted in names.
Examples:
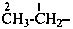
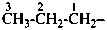
propyl (preferred prefix)
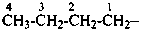
butyl (preferred prefix)
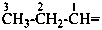
propylidene (preferred prefix)
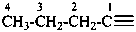
butylidyne (preferred prefix)
Examples:
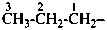
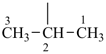
propan-2-yl (preferred prefix)
(not prop-2-yl)
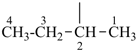
butan-2-yl (preferred prefix)
(not but-2-yl)
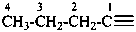
butanylidyne
butylidyne (preferred prefix)
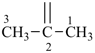
propan-2-ylidene (preferred prefix)
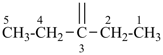
pentan-3-ylidene (preferred prefix)
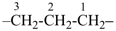
propane-1,3-diyl (preferred prefix)
(not trimethylene)
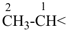
ethane-1,1-diyl ( preferred prefix)
(not ethylidene)
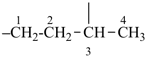
butane-1,3-diyl (preferred prefix)
1-methylpropane-1,3-diyl
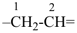
ethan-1-yl-2-ylidene (preferred prefix)
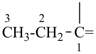
propan-1-yl-1-ylidene ( preferred prefix)
butan-3-yl-1-ylidene (preferred prefix)
butan-2-yl-3-ylidene (preferred prefix)
AsH2-AsH–
diarsanyl (preselected prefix)
SiH3-SiH2–
disilanyl (preselected prefix)
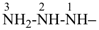
triazan-1-yl (preselected prefix)
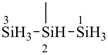
trisilan-2-yl (preselected prefix)
disiloxanyl (preselected prefix)
SiH3-NH-SiH2–
(silylamino)silyl (preselected prefix)
(not disilazan-1-yl;
disilazane is not a recommended parent, hydride,see P-21.2.3.1)
(SiH3)2N–
disilylamino (preselected prefix)
(not disilazan-2-yl;
disilazane is not a recommended parent hydride, see P-21.2.3.1)
bis(silylamino)silyl (preselected prefix)
(not trisilazan-3-yl;
trisilazane is not a recommended parent hydride, see P-21.2.3.1)
H2N-NH–
hydrazinyl (preselected prefix)
diazanyl
H2N-N=
hydrazinylidene (preselected prefix)
diazanylidene
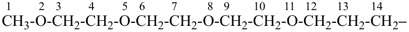
2,5,8,11-tetraoxatetradecan-14-yl (preferred prefix)
Names for monovalent substituent groups derived from cycloalkanes are formed traditionally by method (1) in P-29.2. Names for all other substituent groups derived from saturated cyclic parent hydrides are named by method (2) in P-29.2. Low locants are assigned to free valences ‘yl’, ‘ylidene’, and ‘ylidyne’ in accordance with the numbering of the parent hydride. If there is a choice, the suffixes are assigned low locants, in that order. Suffixes are cited in the order ‘yl’, ‘ylidene’, and ‘ylidyne’. For preferred names see P-57.1.5.1.
Examples:
cyclopentylidene (preferred prefix)
cyclopentanylidene
cyclohexan-1-yl-2-ylidene (preferred prefix)
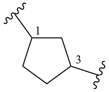
cyclopentane-1,3-diyl (preferred prefix)
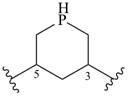
phosphinane-3,5-diyl (preferred prefix)
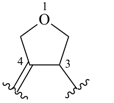
oxolan-3-yl-4-ylidene (preferred prefix)
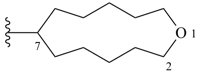
1-oxacyclododecan-7-yl (preferred prefix)
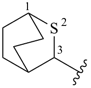
2-thiabicyclo[2.2.2]octan-3-yl (preferred prefix)
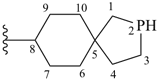
2-phosphaspiro[4.5]decan-8-yl (preferred prefix)
P-29.3.4.1 All mancude rings and ring systems are named by method (2) in P-29.2. When one hydrogen atom is present, there is no difficulty in deriving a monovalent substituent. When no hydrogen atoms are present or when an ‘ylidene’ type substituent group is needed, it is necessary to use ‘added indicated hydrogen’ (see P-14.7). Formally, this method involves the adding of one hydrogen atom to the atom from which the substituent group is derived and another hydrogen atom that can be located on any atom of the ring or ring system. This ‘added indicated hydrogen atom’ is expressed by the symbol H, preceded by a locant denoting its position.
Examples:
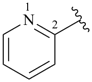
pyridin-2-yl (preferred prefix)
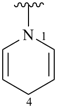
pyridin-1(4H)-yl (preferred prefix)
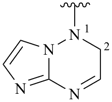
imidazo[1,2-b][1,2,4]triazin-1(2H)-yl (preferred prefix)
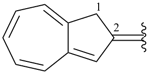
azulen-2(1H)-ylidene (preferred prefix)
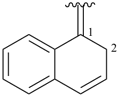
naphthalen-1(2H)-ylidene (preferred prefix)
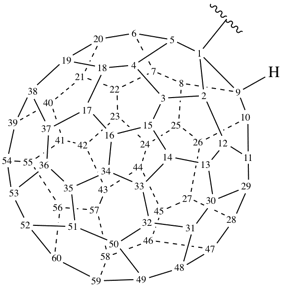
(C60-Ih)[5,6]fulleren-1(9H)-yl (preferred prefix)
Examples:
naphthalene-4a,8a-diyl (preferred prefix)
Examples:
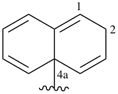
naphthalen-4a(2H)-yl (preferred prefix)
Names of ring assemblies composed of two identical rings or ring systems are formed as described in Section P-28.2, and those derived from ring assemblies composed of three through six identical rings or ring systems are described in P-28.3. Ring assemblies consisting of more than six rings or ring assemblies are named by phane nomenclature as described in P-28.5. Low locants are assigned to ring junctions, then to free valences.
Names of substituent groups derived from ring assemblies are written in two ways:
(1) the suffix ‘yl’ is added to the name biphenyl;Examples:(2) names denoted by locants are placed in brackets; the suffixes ‘yl’ and ‘ylidene’ are added with elision of the final letter ‘e’ in the name of the parent hydride.
[1,1′-biphenyl]-2,4′-diyl (preferred prefix)
[1,1′-bi(cyclohexan)]-4-yl-4′-ylidene (preferred prefix)
[1,1′-bi(cyclohexyl)]-4-yl-4′-ylidene
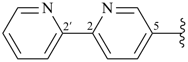
[2,2′-bipyridin]-5-yl (preferred prefix)
[2,2′-bipyridyl]-5-yl
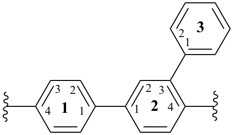
[11,21:23,31-terphenyl]-14,24-diyl (preferred prefix)
[1,1′:3′,1′′-terphenyl]-4,4′-diyl
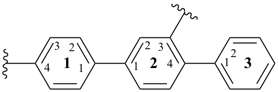
[11,21:24,31-terphenyl]-14,23-diyl (preferred prefix)
[1,1′:4′,1′′-terphenyl]-3′,4-diyl
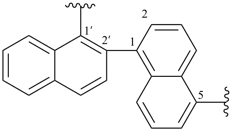
[1,2′-binaphthalene]-1′,5-diyl (preferred prefix)
[1,2′-binaphthyl]-1′,5-diyl
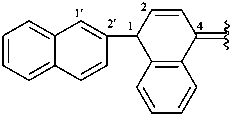
[1,2′-binaphthalen]-4(1H)-ylidene (preferred prefix)
1,2′-binaphthyl]-4(1H)-ylidene
Substituent groups derived from cyclophanes are formed by applying the principles described above.
Examples:
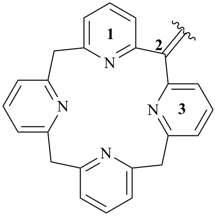
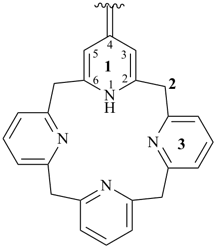
1,3,5,7(2,6)-tetrapyridinacyclooctaphan-14(11H)-ylidene (preferred prefix)
P-29.4.1 Compound substituted substituent groups
A compound substituted substituent group is formed by substituting one or more simple substituents into another simple substituent that is considered as the principal chain. The choice of the principal chain is fully discussed in Section P-44.3. The first criterion to be applied is that the principal chain is the longest chain; it will be applied in the following examples of acyclic compound substituent groups.
Mono-, di-, and polyvalent substituent groups derived from homogeneous chains are named by prefixing the designation of the side chains to the name of the unbranched substituent group possessing the longest possible chain. When a choice is possible, lowest locants are assigned to the side chain. The presence of identical simple substituent groups is indicated by the appropriate multiplying prefix ‘di’, ‘tri’, ‘tetra’, etc.
Names of substituent groups are formed in accordance with P-29.3. Substituent groups of compound substitutive groups must be named by the preferred IUPAC name, as described in Chapter P-5, or preselected name.
Examples:
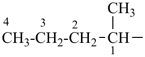
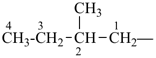
2-methylbutyl (preferred prefix)
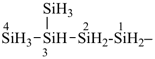
3-silyltetrasilan-1-yl (preselected prefix)
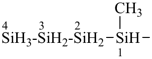
1-methyltetrasilan-1-yl (preferred prefix)
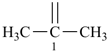
1-methylethylidene
propan-2-ylidene (preferred prefix)
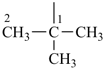
1,1-dimethylethyl (numbering shown)
2-methylpropan-2-yl
tert-butyl (preferred prefix, see P-29.6)
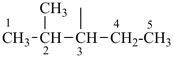
2-methylpentan-3-yl (preferred prefix)
(not 4-methylpentan-3-yl;
locant ‘2’ is lower than ‘4’)
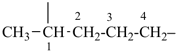
1-methylbutane-1,4-diyl
pentane-1,4-diyl (preferred prefix)
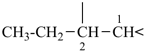
2-ethylethane-1,1,2-triyl
butane-1,1,2-triyl (preferred prefix)
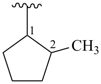
2-methylcyclopentyl (preferred prefix)
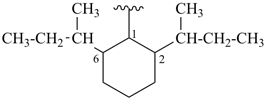
2,6-di(butan-2-yl)cyclohexyl (preferred prefix)
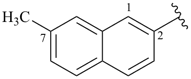
7-methylnaphthalen-2-yl (preferred prefix)
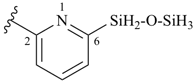
6-disiloxanylpyridin-2-yl (preferred prefix)
Compound catenated substituent groups are used only in multiplicative nomenclature (see P-15.3). Names are formed by adding di- or polyvalent substituent groups to one another, specifically by adding the names of peripheral substituent groups to that of a central substituent group, as prescribed in multiplicative nomenclature (see P-15.3). Substitution of components is allowed when the symmetry of the whole substituent group is preserved.
Examples:
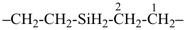
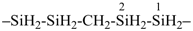
methylenebis(disilane-2,1-diyl) (preferred prefix)
1,4-phenylenebis(methylene) (preferred prefix;
1,4-phenylene and methylene are preferred retained IUPAC prefixes, see P-29.6.1)
cyclohexane-1,4-diylbis(sulfanediyl) (preferred prefix)
cyclohexane-1,4-diylbis(thio)
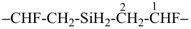
silanediylbis(1-fluoroethane-2,1-diyl) (preferred prefix)
[not silanediylbis(1-fluoroethylene)]
P-29.5.1 Complex substituted substituent groups
Complex substituted substituent groups are formed by substituting an acyclic compound substitutive substituent group into an acyclic substituent group, or into a cyclic substituent group, in accordance with the order of seniority of chains and rings and ring systems (P-44).
Examples:
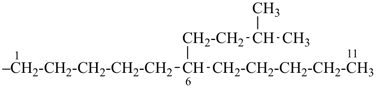
2-(germylmethyl)cyclohexyl (preferred prefix)
Concatenated substituent groups are complex substituent groups when they are composed of three or more components. Substitution is allowed as long as the symmetry of the group is maintained.
Examples:
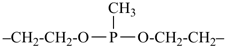
(methylphosphanediyl)bis(oxyethane-2,1-diyl) (preferred prefix)
Three aspects of the use in nomenclature of simple substituent groups derived from parent hydrides with retained names are discussed in this Section:
P-29.6.1 Retained prefixes that are preferred prefixes;P-29.6.1 Retained prefixes that are preferred prefixes
P-29.6.2 Retained prefixes that are not used as preferred prefixes;
P-29.6.3 Retained prefixes no longer recommended as approved prefixes.
The traditional prefixes benzyl, benzylidene, benzylidyne are retained preferred prefixes, but are not to be substituted; however, see P-29.6.2.
In the 1993 Guide (ref. 2), the names ‘benzyl’, ‘benzylidene’, and ‘benzylidyne’ could only be substituted on the ring. In these recommendations, however, for preferred prefixes substitution is not allowed on the ring nor on the side chain, but for general nomenclature, restricted substitution is permitted (see P-29.6.2).
Examples:
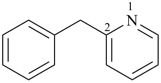
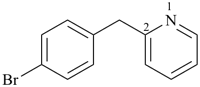
2-(4-bromobenzyl)pyridine
2-[(4-bromophenyl)methyl]pyridine (PIN)
Examples:
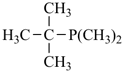
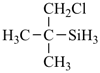
(1-chloro-2-methylpropan-2-yl)silane (PIN)
(2-chloro-1,1-dimethylethyl)silane
Examples:
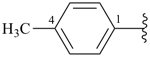
4-methylphenyl (preferred prefix)
2,6-dimethyl-1,4-phenylene (preferred prefix)
P-29.6.2.1 The prefixes benzyl, benzylidene, and benzylidyne are preferred prefixes when unsubstituted. However, these prefixes may be used in general nomenclature when substituted as follows:
(1) unlimited ring substitution;Examples:(2) substitution on the α-position by characteristic atoms or groups that are compulsory substituents (see Table 5.1) or by substituent groups that do not extend the chain.
1-(4-methylphenyl)propyl (preferred prefix)
(not α-ethyl-4-methylbenzyl)
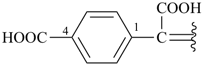
α,4-dicarboxybenzylidene
carboxy(4-carboxyphenyl)methylidene (preferred prefix)
3,4,5-trimethylbenzylidyne
(3,4,5-trimethylphenyl)methylidyne (preferred prefix)
Examples:
1-hydroxypropan-2-ylidene (preferred prefix)
2-hydroxy-1-methylethylidene
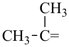
propan-2-ylidene (preferred prefix)
1-methylethylidene
isopropylidene (no substitution allowed)
(C6H5)3C–
trityl (no substitution allowed)
triphenylmethyl (preferred prefix)
(4-methylphenyl)di(phenyl)methyl (preferred prefix)
Examples:
–CHCl-CHCl–
1,2-dichloroethylene
1,2-dichloroethane-1,2-diyl (preferred prefix)
2-adamantyl (also 1-isomer)
adamantan-2-yl (preferred prefix)
tricyclo[3.3.1.13,7]decan-2-yl
2-anthryl (also 1- and 9-isomers)
anthracen-2-yl (preferred prefix)
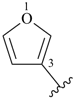
3-furyl (also 2-isomer)
furan-3-yl (also 2-isomer; preferred prefixes)
7-isoquinolyl (also 1-, 3-, 4-, 5-, 6- and 8-isomers)
isoquinolin-7-yl (also 1-, 3-, 4-, 5-, 6- and 8-isomers; preferred prefixes)
2-naphthyl (also 1-isomer)
naphthalen-2-yl (also 1-isomer; preferred prefixes)
9-phenanthryl (also 1-, 2-, 3- and 4-isomers)
phenanthren-9-yl (also 1-, 2-, 3- and 4-isomers; preferred prefixes)
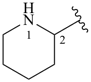
2-piperidyl (also 1-, 3- and 4-isomers)
piperidin-2-yl (also 1-, 3- and 4-isomers; preferred prefixes)
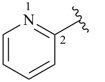
2-pyridyl (also 3- and 4-isomers)
pyridin-2-yl (also 3- and 4-isomers; preferred prefixes)
2-quinolyl (also 3-, 4-, 5-, 6-, 7- and 8-isomers)
quinolin-2-yl (also 3-, 4-, 5-, 6-, 7- and 8-isomers; preferred prefixes)
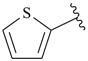
2-thienyl (also 3-isomer)
thiophen-2-yl (also 3-isomer; preferred prefixes)
o-tolyl (also m- and p-isomers; no substitution allowed)
2-methylphenyl (also 3- and 4-isomers; preferred prefixes)
Trivial, common, and traditional prefixes have always been an integral part of organic nomenclature. However, as systematic nomenclature develops and becomes widely used, many of these prefixes fall by the wayside. Accordingly, each set of IUPAC recommendations contains fewer of these traditional prefixes. These recommendations are no different. The following prefixes were still contained in the 1993 recommendations (ref. 2) but are NOT recommended in these recommendations; their systematic prefixes based on these recommendations are given as ‘preferred prefixes’.
(C6H5)2CH–
(not benzhydryl)
diphenylmethyl (preferred prefix)
(CH3)2CH-CH2–
(not isobutyl)
2-methylpropyl (preferred prefix)
CH3-CH2-CH(CH3)–
(not sec-butyl)
butan-2-yl (preferred prefix)
1-methylpropyl
(CH3)2CH-CH2-CH2–
(not isopentyl)
3-methylbutyl (preferred prefix)
CH3-CH2-C(CH3)2–
(not tert-pentyl)
2-methylbutan-2-yl (preferred prefix)
1,1-dimethylpropyl
(CH3)3C-CH2–
(not neopentyl)
2,2-dimethylpropyl (preferred prefix)
[not furfuryl (2-isomer only)]
(furan-2-yl)methyl (preferred prefix)
[not thenyl (2-isomer only)]
(thiophen-2-yl)methyl (preferred prefix)
2-thienylmethyl
Return to:
Blue Book home page.