Division VIII Chemical Nomenclature and Structure Representation Division
(P-66 to P-69)
(continued from P-60 to P-65)
P-60 Introduction
P-61 Substitutive nomenclature: prefix mode
P-62 Amines and imines
P-63 Hydroxy compounds, ethers, peroxols, peroxides and chalcogen analogues
P-64 Ketones, pseudoketones and heterones, and chalcogen analogues
P-65 Acids and derivatives
P-66 Amides, hydrazides, nitriles, aldehydes
P-67 Oxoacids used as parents for organic compounds
P-68 Nomenclature of other classes of compounds
P-69 Organometallic compounds
P-66.0 IntroductionP-66.0 INTRODUCTION
P-66.1 Amides
P-66.2 Imides
P-66.3 Hydrazides
P-66.4 Amidines, amidrazones, hydrazidines, and amidoximes (amide oximes)
P-66.5 Nitriles
P-66.6 Aldehydes
The classes dealt with in this Section have in common the fact that their retained names are derived from those of acids by changing the ‘ic acid’ ending to a class name, for example ‘amide’, ‘ohydrazide’, ‘nitrile’, or ‘aldehyde’. Their systematic names are formed substitutively by the suffix mode using one of two types of suffix, one that includes the carbon atom, for example, ‘carbonitrile’ for –CN, and one that does not, for example, ‘-nitrile’ for –(C)N. Amidines are named as amides, hydrazidines as hydrazides, and amidrazones as amides or hydrazides.
P-66.1 AMIDES
P-66.1.0 IntroductionP-66.1.0 Introduction
P-66.1.1 Primary amides
P-66.1.2 Secondary and tertiary amides
P-66.1.3 ‘Hidden’ amides
P-66.1.4 Chalcogen analogues of amides
P-66.1.5 Lactams, lactims, sultams, and sultims
P-66.1.6 Amides derived from carbonic, cyanic, and the di- and polycarbonic acids
P-66.1.7 Polyfunctional amides
Amides are derivatives of organic oxoacids in which each hydroxy group has been replaced by an amino or substituted amino group. Chalcogen replacement analogues are called thio-, seleno-, and telluroamides. Compounds having one, two, or three acyl groups on a single nitrogen atom are generically included.
P-66.1.1 Primary amides
P-66.1.1.1 CarboxamidesP-66.1.1.1 Carboxamides
P-66.1.1.2 Sulfonamides, sulfinamides, and related selenium and tellurium amides
P-66.1.1.3 Substitution of primary amides
P-66.1.1.4 Amides denoted as prefixes
Names of carboxamides are formed in two ways:
P-66.1.1.1.1 Substitutive nomenclatureP-66.1.1.1.1 Amide names formed by substitutive nomenclature
P-66.1.1.1.2 Modification of retained names for acids
P-66.1.1.1.1.1 Alicyclic mono- and diamides are named substitutively by adding the suffix ‘amide’, to the appropriate parent hydride name, with elision of the final letter ‘e’ before ‘a’. The multiplying prefix ‘di’ is used to name diamides.
Examples:
H2N-CO-CH2-CH2-CH2-CO-NH2
pentanediamide (PIN)
Example:
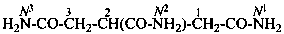
Examples:
H2N-NH-CO-NH2
hydrazinecarboxamide (PIN)
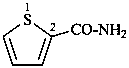
thiophene-2-carboxamide (PIN)
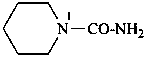
piperidine-1-carboxamide (PIN)
Names of amides derived from carboxylic acids listed in P-65.1.1 are formed by changing the ‘ic acid’ or ‘-oic acid’ ending of the retained names of carboxylic acids into ‘amide’. Names of amides formed by this method are either preferred IUPAC names or names for use in general nomenclature according to the status of the corresponding acid; structures can be substituted in the same way as indicated for the corresponding acids (see P-65.1.1).
P-66.1.1.1.2.1 Only the following four retained names are preferred IUPAC names and can be substituted. The name ‘oxamide’ is a contracted name from ‘oxalamide’ and substitution on the nitrogen atoms is allowed.
C6H5-CO-NH2
benzamide (PIN)
H2N-CO-CO-NH2
oxamide (PIN)
NC-NH2
cyanamide
(PIN, see P-66.1.6.2)
Examples:
Cl-CO-NH2
carbonochloridic amide (PIN)
(not 1-chloroformamide)
Examples:
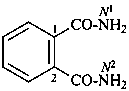
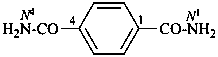
benzene-1,4-dicarboxamide (PIN)
terephthalamide
Examples:
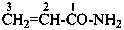
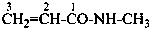
N-methylprop-2-enamide (PIN)
(not N-methylacrylamide;
substitution is not allowed on acrylamide)
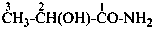
2-hydroxypropanamide (PIN)
lactamide
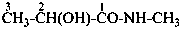
2-hydroxy-N-methylpropanamide (PIN)
(not N-methyllactamide)
Examples:
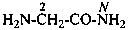
2-aminoacetamide
glycinamide
These suffixes may be assigned to any position of a parent hydride.
–SO2-NH2 sulfonamide (preselected suffix) –SO-NH2 sulfinamide (preselected suffix) –SeO2-NH2 selenonamide (preselected suffix) –SeO-NH2 seleninamide (preselected suffix) –TeO2-NH2 telluronamide (preselected suffix) –TeO-NH2 tellurinamide (preselected suffix)
Examples:
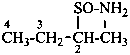
butane-2-sulfinamide (PIN)
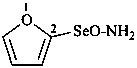
furan-2-seleninamide (PIN)
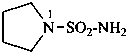
pyrrolidine-1-sulfonamide (PIN)
P-66.1.1.3.1 N-SubstitutionP-66.1.1.3.1 N-Substitution
P-66.1.1.3.2 Hydroxamic acids
P-66.1.1.3.3 Amic acids
P-66.1.1.3.4 Anilides
P-66.1.1.3.5 General substitution of amides
P-66.1.1.3.1.1 Substituted primary amides, with general structures such as R-CO-NHR′ and R-CO-NR′R′′, and the corresponding amides derived from chalcogen acids are named by citing the substituents R′ and R′′ as prefixes preceded by the locant N when one amide group is present. In di- and polyamides, except for geminal diamides, N locants with superscripted arabic numbers, which are the locants of the parent structure, are used to differentiate the nitrogen atoms, for example, N1, N3, etc. (see also P-62.2.4.1.2). The recommended N locants for geminal diamides are discussed in P-66.1.1.3.1.2.
Superscript arabic numbers are now used to differentiate the nitrogen atoms of diamides, where primes (′), double primes (′′), triple primes (′′′), etc. were formerly used.
N-Substitution of primary amides is not allowed when amides having retained names are designated as not substitutable.
Examples:
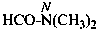
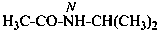
N-(propan-2-yl)acetamide (PIN)
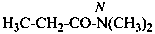
N,N-dimethylpropanamide (PIN)
(not N,N-dimethylpropionamide;
substitution is not allowed on propionamide)
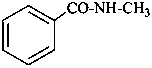
N-methylbenzamide (PIN)
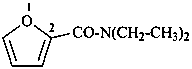
N,N-diethylfuran-2-carboxamide (PIN)
N,N-diethyl-2-furamide
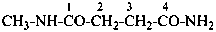
N1-methylbutanediamide (PIN)
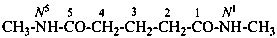
N1,N5-dimethylpentanediamide (PIN)
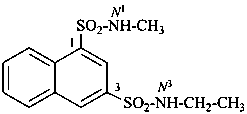
N3-ethyl-N1-methylnaphthalene-1,3-disulfonamide (PIN)
When geminal carboxamide groups are present, the locants N, N′, etc. are used in association with the numerical locant indicating the position of the groups on a chain or ring. Lowest locants are assigned to the most substituted group; when there is a choice, lowest locants are assigned to the first cited N-substituent.
Examples:
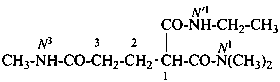
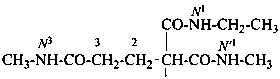
N1-ethyl-N′1,N3-dimethylpropane-1,1,3-tricarboxamide (PIN)
Hydroxamic acids have the generic structure R-CO-NH-OH and are named as N-hydroxy amides (see P-65.1.3.4). The suffixes ‘hydroxamic acid’ and ‘carbohydroxamic acid’ but may be used in general nomenclature.
Examples:
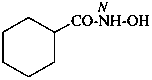
N-hydroxycyclohexanecarboxamide (PIN)
cyclohexanecarbohydroxamic acid
Amic acids are derivatives of dicarboxylic acids having a retained name and when one carboxylic group has been changed to a carboxamide group. Preferred IUPAC names for amic acids are generated by method (1) in P-66.1.1.4.1.1. They may also be named by methods (2) and (3) and by replacing the ‘ic acid’ ending in the retained name by ‘amic acid’ (see P-65.1.6.1).
Examples:
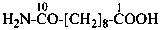
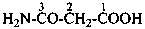
(1) 3-amino-3-oxopropanoic acid (PIN)
(2) carbamoylacetic acid
(3) (aminocarbonyl)acetic acid
malonamic acid (see P-65.1.6.1)
N-Phenyl derivatives of primary amides are called ‘anilides’ and may be named using the term ‘anilide’ in place of ‘amide’ in systematic or retained names of amides. The locants for substituents in the N-phenyl ring of anilides are primed numbers. However, names expressing N-substitution by a phenyl group on an amide are preferred IUPAC names.
Examples:
CH3-CO-NH-C6H5
N-phenylacetamide (PIN)
acetanilide
CH3-[CH2]4-CO-NH-C6H5
N-phenylhexanamide (PIN)
hexananilide
C6H5-CO-N(CH3)-C6H5
N-methyl-N-phenylbenzamide (PIN)
N-methylbenzanilide
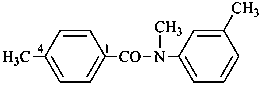
N,4-dimethyl-N-(3-methylphenyl)benzamide (PIN)
N,3′,4-trimethylbenzanilide
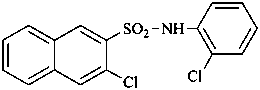
3-chloro-N-(2-chlorophenyl)naphthalene-2-sulfonamide (PIN)
2′,3-dichloronaphthalene-2-sulfonanilide
Substitution of amides is expressed by prefixes; numerical, N, and N′ locants are used as required. N-Substitution of amides follows the substitution rules for carboxylic acids described in P-65.1.2.4.
Examples:
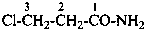
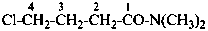
4-chloro-N,N-dimethylbutanamide (PIN)
(not 4-chloro-N,N-dimethylbutyramide;
no substitution on butyramide)
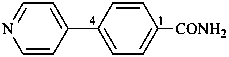
4-(pyridin-4-yl)benzamide (PIN)
4-(4-pyridyl)benzamide
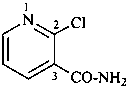
2-chloropyridine-3-carboxamide (PIN)
(not 2-chloronicotinamide)
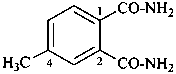
4-methylbenzene-1,2-dicarboxamide (PIN)
4-methylphthalamide
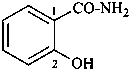
2-hydroxybenzamide (PIN)
(not salicylamide)
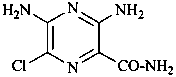
3,5-diamino-6-chloropyrazine-2-carboxamide (PIN)
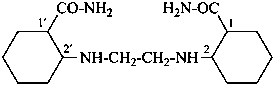
2,2′-[ethane-1,2-diylbis(azanediyl)]di(cyclohexane-1-carboxamide) (PIN)
Two different substituent groups can be derived from amides and expressed as prefixes in the presence of a characteristic group having seniority for citation as suffix:
P-66.1.1.4.1 Substituents of the types –CO-NH2 and –CO-CO-NH2P-66.1.1.4.1 Substituents of the types –CO-NH2 and –CO-CO-NH2.
P-66.1.1.4.2 Substituents of the types –SO2-NH2 , –SO-NH2 and their selenium and tellurium analogues
P-66.1.1.4.3 Substituents of the types –NH-CO-R and –NH-SO2-R
P-66.1.1.4.4 Substituent groups R-CO-N< and R-CO-N=, or R-SO2-N< and R-SO2-N= (and selenium and tellurium analogues)
P-66.1.1.4.5 Substituent groups derived from oxamide, H2N-CO-CO-NH2
P-66.1.1.4.1.1 In the presence of a characteristic group having priority for citation as suffix or if all carbamoyl groups cannot be included in the suffix, the –CO-NH2 group is named in three different ways:
(1) by using the two prefixes ‘amino’ and ‘oxo’ to denote such groups on terminal atoms of carbon chains having more than one carbon atom;For generation of IUPAC preferred names, method (1) is preferred for chains and method (2) for rings and ring systems, heterogeneous chains, and on nonterminal atoms of carbon chains. Carbamoyl and amino groups may be substituted in the normal way.(2) by using the acyl group name ‘carbamoyl’ (see P-65.2.1.5);
(3) by using the prefix ‘aminocarbonyl’.
Examples:
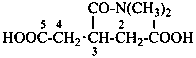
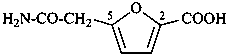
(1) 5-(2-amino-2-oxoethyl)furan-2-carboxylic acid (PIN)
(2) 5-(carbamoylmethyl)furan-2-carboxylic acid
(3) 5-[(aminocarbonyl)methyl]furan-2-carboxylic acid
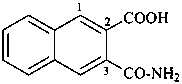
(2) 3-carbamoylnaphthalene-2-carboxylic acid (PIN)
3-carbamoyl-2-naphthoic acid
(3) 3-(aminocarbonyl)naphthalene-2-carboxylic acid
3-(aminocarbonyl)-2-naphthoic acid
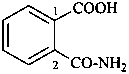
(2) 2-carbamoylbenzoic acid (PIN)
(3) 2-(aminocarbonyl)benzoic acid
phthalamic acid (see P-65.1.6.1)
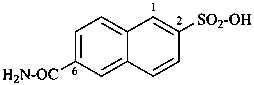
(2) 6-carbamoylnaphthalene-2-sulfonic acid (PIN)
(3) 6-(aminocarbonyl)naphthalene-2-sulfonic acid
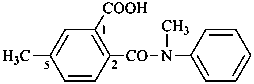
(2) 5-methyl-2-[methyl(phenyl)carbamoyl]benzoic acid (PIN)
(3) 5-methyl-2-[(N-methylanilino)carbonyl]benzoic acid
5-methyl-2-{[methyl(phenyl)amino]carbonyl}benzoic acid
Examples:
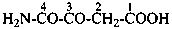
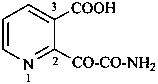
(2) 2-oxamoylpyridine-3-carboxylic acid (PIN)
(3) 2-(aminooxalyl)pyridine-3-carboxylic acid
In the presence of a characteristic group having priority for citation as suffix, the groups –SO2-NH2, –SO-NH2 and related selenium and tellurium groups are named in two ways corresponding to methods (2) and (3) for the –CO-NH2 group above in P-66.1.1.4.1:
(2) by using the acyl group ‘sulfamoyl’ (for sulfonamides only) (see P-65.3.2.3);For –SO2-NH2, method (2) generates preferred IUPAC names; for all other groups method (3) is the only method for generation of preferred IUPAC names.(3) by using the prefixes ‘amino...sulfonyl’, ‘amino...sulfinyl’, ‘amino...selenonyl’, ‘amino...seleninyl’, ‘amino...telluronyl’, or ‘amino...tellurinyl’.
Examples:
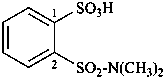
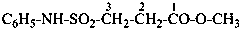
methyl 3-(phenylsulfamoyl)propanoate (PIN)
(3) methyl 3-[(phenylamino)sulfonyl]propanoate
methyl 3-(anilinosulfonyl)propanoate
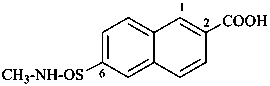
(3) 6-[(methylamino)sulfinyl]naphthalene-2-carboxylic acid (PIN)
(3) 6-[(methylamino)sulfinyl]-2-naphthoic acid,
When a group having preference for citation as a principal characteristic group is present, the group R-CO-NH–, or R-SO2-NH– (and selenium and tellurium analogues) of an N-substituted amide is named in two ways:
(1) substitutively, by using a prefix formed by changing the final letter ‘e’ in the complete name of the amide to ‘o’, thus changing the suffixes ‘amide’ and ‘carboxamide’ into ‘amido’ and ‘carboxamido’, respectively, ‘diamide’ to ‘diamido’ or ‘sulfonamide’ to ‘sulfonamido’, etc.;Method (1) generates preferred IUPAC names.(2) substitutively, by using ‘acylamino’ prefixes formed by substituting the substituent group ‘amino’ with the name of the acyl group.
Examples:
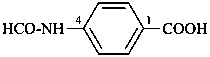
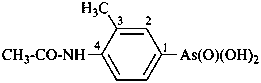
(1) (4-acetamido-3-methylphenyl)arsonic acid (PIN)
(2) [4-(acetylamino)-3-methylphenyl]arsonic acid
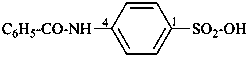
(1) 4-benzamidobenzene-1-sulfonic acid (PIN)
(2) 4-(benzoylamino)benzene-1-sulfonic acid
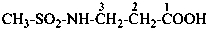
(1) 3-(methanesulfonamido)propanoic acid (PIN)
(2) 3-[(methanesulfonyl)amino]propanoic acid
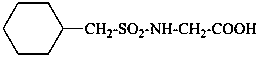
N-(cyclohexylmethanesulfonyl)glycine
(1) (1-cyclohexylmethanesulfonamido)acetic acid
(2) {[(cyclohexylmethyl)sulfonyl]amino}acetic acid
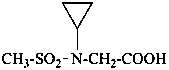
N-cyclopropyl-N-(methanesulfonyl)glycine
(1) (N-cyclopropylmethanesulfonamido)acetic acid
(2) [(cyclopropyl(methanesulfoyl)amino]acetic acid
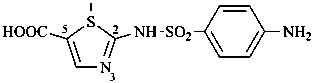
(1) 2-(4-aminobenzene-1-sulfonamido)-1,3-thiazole-5-carboxylic acid (PIN)
(not 2-sulfanilamidothiazole-5-carboxylic acid;
sulfanilic acid
is not a retained name)
(2) 2-{[(4-aminophenyl)sulfonyl]amino}-1,3-thiazole-5-carboxylic acid
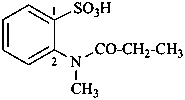
(1) 2-(N-methylpropanamido)benzene-1-sulfonic acid (PIN)
(2) 2-[methyl(propanoyl)amino]benzene-1-sulfonic acid
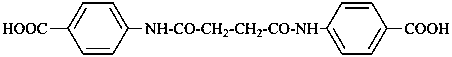
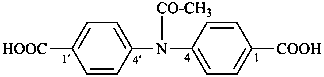
(1) 4,4′-butanediamidodibenzoic acid (PIN)
(2) 4,4′-[butanedioylbis(azanediyl)]dibenzoic acid
4,4′-[1,4-dioxobutane-1,4-diylbis(azanediyl)]dibenzoic acid
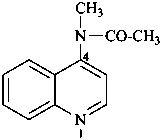
N-methyl-N-(quinolin-4-yl)acetamide (PIN)
(not 4-(N-methylacetamido)quinoline)
{not 4-[acetyl(methyl)amino]quinoline}
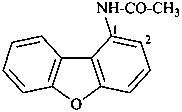
N-(dibenzo[b,d]furan-1-yl)acetamide (PIN)
(not 1-acetamidodibenzofuran)
[not 1-(acetylamino)dibenzofuran]
When an amide is the principal function, it must be named as such. The method of considering amides as substituents on polycyclic ring systems, described in the 1993 Recommendations (ref. 2), should be avoided, even in general nomenclature (see also P-66.1.3).
P-66.1.1.4.4 Substituent groups R-CO-N< and R-CO-N= , or R-SO2-N< and R-SO2-N= (and selenium and tellurium analogues)
When a group having preference for citation as a principal characteristic group is present, the groups R-CO-N< and R-CO-N=, or R-SO2-N< and R-SO2-N= (and selenium and tellurium analogues) of an N-substituted amide are named by combining acyl group names with those of the appropriate nitrogen substituent groups, azanediyl and imino, respectively.
Examples:
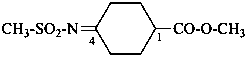
methyl 4-[(methanesulfonyl)imino]cyclohexane-1-carboxylate (PIN)
P-66.1.1.4.5.1 Prefixes for the group H2N-CO-CO-NH– are ‘oxamoylamino’ (preferred prefix) or ‘amino(oxo)acetamido’. The preferred prefix for the group H2N-CO-CO-N= is ‘oxamoylimino’.
Example:
Concatenated preferred prefixes for the groups –HN-CO-CO-NH–, >N-CO-CO-N<, and =N-CO-CO-N= are ‘oxalylbis(azanediyl)’, ‘oxalyldinitrilo’, and ‘oxalylbis(azanylylidene)’, respectively. The preferred prefix for the group H2N-CO-CO-N< is ‘oxamoylazanediylְ.
Example:
Amides having general formulas (R-CO)2NH, (R-SO2)2NH, etc., and (R-CO)3N, (R-SO2)3N, etc., respectively, are named as N-acyl derivatives of the senior primary amide or preferred prefix. Names based on the substitution of the parent hydride ‘azane’ or the pseudo parent hydride ‘amine’ by acyl groups, for example, diacetylazane or diacetylamine, as recommended in the 1993 Recommendations (ref. 2) are not included in these recommendations, nor are trivial names such as diacetamide, triacetamide, dibenzamide, and tribenzamide.
Examples:
(CH3-CO)2N–
N-acetylacetamido (preferred prefix)
diacetylamino
(not diacetylazanyl)
(not diacetamido)
C6H5-CO-NH-CO-CH3
N-acetylbenzamide (PIN)
[not acetyl(benzoyl)azane]
[not acetyl(benzoyl)amine]
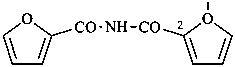
N-(furan-2-carbonyl)furan-2-carboxamide (PIN)
[not di(furan-2-carbonyl)azane]
[not di(furan-2-carbonyl)amine]
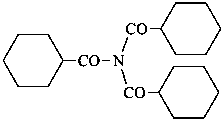
N,N-di(cyclohexanecarbonyl)cyclohexanecarboxamide (PIN)
[not tri(cyclohexanecarbonyl)azane]
[not tri(cyclohexanecarbonyl)amine]
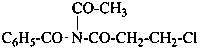
N-acetyl-N-(3-chloropropanoyl)benzamide (PIN)
[not acetyl(benzoyl)(3-chloropropanoyl)azane]
[not acetyl(benzoyl)(3-chloropropanoyl)amine]
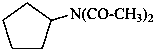
N-acetyl-N-cyclopentylacetamide (PIN)
[not diacetyl(cyclopentyl)azane]
[not diacetyl(cyclopentyl)amine]
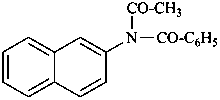
N-acetyl-N-(naphthalen-2-yl)benzamide (PIN)
[not acetyl(benzoyl)(naphthalen-2-yl)azane]
[not acetyl(benzoyl)(naphthalen-2-yl)amine]
An N-acyl group attached to a nitrogen atom of a heterocyclic system has been called a ‘hidden amide’, i.e., an amide that cannot be named as such by accepted substitutive principles. The traditional way to name such compounds by using acyl groups as substituents on the nitrogen atom of the heterocyclic system is allowed but only in general nomenclature. Such compounds are now considered as pseudoketones (see P-64.3) and preferred IUPAC names are constructed accordingly.
An N-acyl group attached to a nitrogen atom of a heterocyclic system is now preferably named as a pseudoketone (see P-64.1.2.1, P-64.3) and not as an acyl substituent on the nitrogen as in previous recommendations; the latter method can be used in general nomenclature.
Examples:
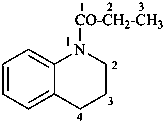
1-(3,4-dihydroquinolin-1(2H)-yl)propan-1-one (PIN)
1-propanoyl-1,2,3,4-tetrahydroquinoline
1-propionyl-1,2,3,4-tetrahydroquinoline
Chalcogen analogues of amides are named systematically. Prefixes, such as ‘thio’, modifying retained names are no longer recommended for preferred names.
P-66.1.4.1 Names of chalcogen analogues of primary amides
P-66.1.4.1.1 Names are formed by using suffixes modified by functional replacement nomenclature using prefixes and infixes.
Examples:
| –(C)S-NH2 | -thioamide (preferred suffix) | |
| –CS-NH2 | -carbothioamide (preferred suffix) | |
| –S(O)(S)-NH2 | -sulfonothioamide (preselected suffix) | |
| –S(S)(S)-NH2 | -sulfonodithioamide (preselected suffix) | |
| –S(S)-NH2 | -sulfinothioamide (preselected suffix) |
Examples:
CH3-CS-NH2
ethanethioamide (PIN)
thioacetamide
C6H5-CS-NH2
benzenecarbothioamide (PIN)
thiobenzamide
H2N-CS-CH2-CH2-CS-NH2
butanedithioamide (PIN)
CH3-[CH2]4-CS-NH2
hexanethioamide (PIN)
CH3-CH2-CS-NH2
propanethioamide (PIN)
(not thiopropionamide)
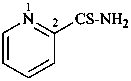
pyridine-2-carbothioamide (PIN)
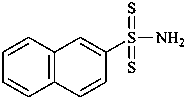
naphthalene-2-sulfonodithioamide (PIN)
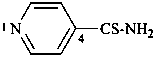
pyridine-4-carbothioamide (PIN)
(not thioisonicotinamide)
Names are formed by the addition of appropriate prefixes to names described in P-65.1.7.2.3
Examples:
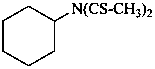
N-cyclohexyl-N-(ethanethioyl)ethanethioamide (PIN)
N-cyclohexyl-N-(thioacetyl)thioacetamide
CH3-CH2-CS-NH-CO-CH3
N-(propanethioyl)acetamide (PIN)
Names are formed as described in P-64.6.1 for chalcogen derivatives of pseudoketones.
Example:
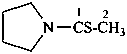
(1) by a prefix formed by changing the final letter ‘e’ in the name of the amide into ‘o’;Examples:(2) by the appropriate prefixes, such as amino, in conjunction with sulfanylidene or thioxo, as well as carbonothioyl (not thiocarbonyl) for –CS– or carbamothioyl (not thiocarbamoyl) for –CS-NH2.
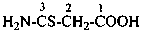
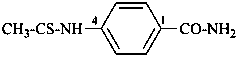
4-(ethanethioamido)benzamide (PIN)
4-[(ethanethioyl)amino]benzamide
4-(thioacetamido)benzamide
CH3-S(S)-NH-CH2-COOH
[(methanesulfinothioyl)amino]acetic acid (PIN)
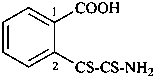
2-[amino(sulfanylidene)ethanethioyl]benzoic acid (PIN)
P-66.1.5.1 Lactams and lactims
Intramolecular amides of amino carboxylic acids, –CO-NH–, are called ‘lactams’ and their tautomers, –C(OH)=N–, are ‘lactims’. Lactams are named in two ways:
(1) as heterocyclic pseudoketones;Method (1) generates preferred IUPAC names.(2) by substituting ‘lactam’ for the ‘ic acid’ ending of a systematic ‘oic acid’ name for the parent acid without the amino substituent, and inserting a locant designating the position of the amino group between the ‘o’ and ‘lactam’. Lactims are named in the same way, using ‘lactim’ in place of ‘lactam’.
Examples:
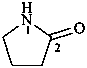
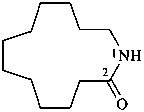
1-azacyclotridecan-2-one (PIN)
dodecano-12-lactam
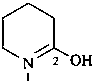
3,4,5,6-tetrahydropyridin-2-ol (PIN)
pentano-5-lactim
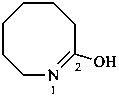
3,4,5,6,7,8-hexahydroazocin-2-ol (PIN)
1,2-didehydroazocan-2-ol
heptano-7-lactim
P-66.1.5.2.1 Intramolecular amides of amino sulfonic acids are called ‘sultams’ and may be named in three ways
(1) as heterocyclic heterones;Method (1) leads to preferred IUPAC names.(2) by citing the term ‘sultam’ denoting the cyclic –NH-SO2– group after the name of the appropriate parent hydride preceded by a pair of locants describing the points of attachment of the sulfonyl group and the nitrogen atom, respectively; the locant of the sulfonyl group is cited first, and, if there is a choice, is the lower locant. Multiplying prefixes and pairs of locants separated by a colon are used to indicate two or more sultam rings;
(3) as heterocycles and, according to functional class nomenclature, using the class term ‘oxide’.
Examples:
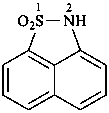
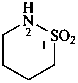
(1) 1λ6,2-thiazinane-1,1-dione (PIN)
(3) 1,2-thiazinane 1,1-dioxide
(2) butane-1,4-sultam
Examples:
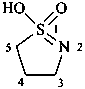
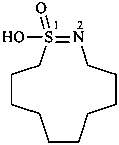
(1) 1-hydroxy-1λ6-thia-2-azacyclododec-1-en-1-one (PIN)
(3) 1-hydroxy-1λ4-thia-2-azacyclododec-1-ene 1-oxide
(2) decane-1,10-sultim
Cyclic amides of amino sulfinic acids and their tautomers are named as heterocyclic compounds.
Examples:
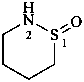
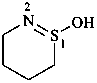
3,4,5,6-tetrahydro-1λ4,2-thiazin-1-ol (PIN)
P-66.1.6.1 Amides derived from carbonic acid and related compounds
P-66.1.6.1.1 Urea and its substitutive derivativesP-66.1.6.1.1 Urea and its substitutive derivatives
P-66.1.6.1.2 Isourea and its derivatives
P-66.1.6.1.3 Chalcogen analogues of urea and isourea
P-66.1.6.1.4 Condensed ureas
P-66.1.6.1.1.1 The compound H2N-CO-NH2 has the retained name ‘urea’, which is the preferred IUPAC name, with locants N and N′, as shown above the structure below. The systematic name is ‘carbonic diamide’. The locants 1, 2, and 3 have been used in the past and may be used in general nomenclature.
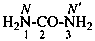
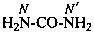
carbonic diamide
Numerical locants for urea are no longer used in the IUPAC preferred name.
P-66.1.6.1.1.2 Derivatives of urea formed by substitution on the nitrogen atom(s) are named as substitution products in accordance with the seniority order of urea that is ranked as an amide of carbonic acid. Amides of cyanic and the di- and polycarbonic acids follow the same seniority as the corresponding acid (see P-42.2).
Examples:
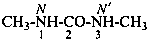
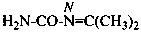
N-(propan-2-ylidene)urea (PIN)
isopropylideneurea
N-(propan-2-ylidene)carbonic diamide
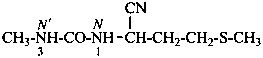
N-[1-cyano-3-(methylsulfanyl)propyl]-N′-methylurea (PIN)
N-[1-cyano-3-(methylsulfanyl)propyl]-N′-methylcarbonic diamide
The prefixes ‘ureido’ and ‘ureylene’ are no longer acceptable in IUPAC nomenclature. The prefixes ‘carbamoylamino’ and ‘carbonylbis(azanediyl)’, respectively are recommended for preferred IUPAC names.
–HN-CO-NH–
carbonylbis(azanediyl)
(preferred prefix; for use in multiplicative nomenclature)
(not ureylene)
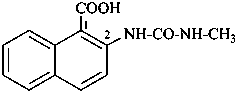
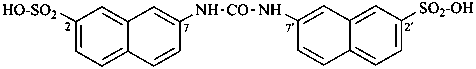
7,7′-[carbonylbis(azanediyl)]di(naphthalene-2-sulfonic acid) (PIN)
[not 7,7′-ureylenedi(naphthalene-2-sulfonic acid)]
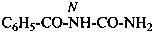
N-carbamoylbenzamide (PIN)
N-(aminocarbonyl)benzamide
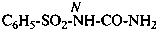
N-carbamoylbenzenesulfonamide (PIN)
N-(aminocarbonyl)benzenesulfonamide
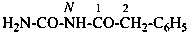
N-carbamoyl-2-phenylacetamide (PIN)
N-(aminocarbonyl)-2-phenylacetamide
Two carboxylic acids are related to urea; have been known as ‘allophanic acid’ for H2N-CO-NH-COOH and ‘hydantoic acid’ for H2N-CO-NH-CH2-COOH. These names are no longer recommended. Preferred IUPAC names for these two acids and their derivatives are formed systematically.
Examples:
H2N-CO-NH-CO–
carbamoylcarbamoyl (preferred prefix)
[(aminocarbonyl)amino]carbonyl
H2N-CO-NH-CH2-COOH
N-carbamoylglycine
(carbamoylamino)acetic acid
Amides are ranked in the same way as the corresponding acids (see P-42). Thus, in substitutive nomenclature, amides from carboxylic acids, including formamide, are senior to urea.
Examples:
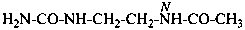
H2N-CO-NH-CH2-CH2-CH2-NH-CHO
N-[3-(carbamoylamino)propyl]formamide (PIN)
[not N-(3-formamidopropyl)urea
nor N-[3-(formylamino)propyl]urea;
formamide is preferred to urea, see P-41]
P-66.1.6.1.2.1 The imidic acid tautomer of urea, H2N-C(OH)=NH, is named ‘carbamimidic acid’, a shortened form of the systematic functional replacement name ‘carbonamidimidic acid’. The name ‘isourea’ is no longer recommended, but is retained as a class name. In preferred IUPAC names, derivatives of carbamimidic acid are named using the locants N and N′. Since the name isourea is no longer recommended, even for general nomenclature, no numerical locants used in previous recommendations are needed. When the position of the double bond is unknown, only the locant N is used.
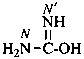
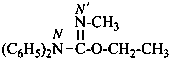
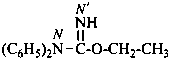
ethyl N,N-diphenylcarbamimidate (PIN)
(not O-ethyl-N,N-diphenylisourea)

ethyl N-phenylcarbamimidate (PIN)
(not O-ethyl-N-phenylisourea)
The prefixes 1-isoureido and 3-isoureido are no longer recommended.
Example:
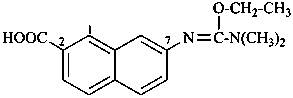
P-66.1.6.1.3.1 Chalcogen analogues of urea are named by functional replacement nomenclature using the prefixes ‘thio’, ‘seleno’, and ‘telluro’. Preferred IUPAC names use the letter locants N, and N′. Numerical locants may be used for thiourea in general nomenclature.
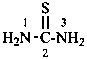
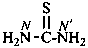
thiourea (PIN)
Numerical locants are no longer used for thiourea in the IUPAC preferred name.
Example:
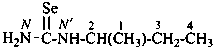
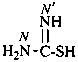
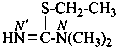
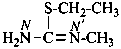
ethyl N′-methylcarbamimidothioate (PIN)
(not S-ethyl-N′-methylisothiourea)
| H2N-CS-NH– | carbamothioylamino (preferred prefix) [amino(sulfanylidene)methyl]amino- | |
| HN=C(SH)-NH– | (C-sulfanylcarbonimidoyl)amino (preferred prefix) [imino(sulfanyl)methyl]amino- | |
| H2N-C(SH)=N– | [amino(sulfanyl)methylidene]amino (preferred prefix) |
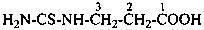
Condensed ureas, H2N-[CO-NH]n-H, where n = 2, 3, or 4, are named systematically as diamides of imidodicarbonic acid, diimidotricarbonic acid, and triimidotetracarbonic acid, etc., functional replacement names derived from the corresponding di- or polycarbonic acids. The names biuret, triuret, etc., are no longer recommended as preferred IUPAC names. Chalcogen analogues are described by functional replacement prefixes alphabetized along with ‘imido’ in front of the corresponding di- or polycarbonic acid. Locants, as shown above the structures, below, are used to indicate the positions of substituents and functional replacement prefixes, where needed. Preferred IUPAC names use these locants which are also prescribed for the amides of imidopolycarbonic acids (see P-66.4.1.2.2). The full numerical numbering system used previously and shown below the structures may be used in general nomenclature.
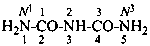
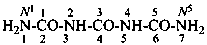
2,4-diimidotricarbonic diamide (PIN)
triuret
Numerical locants for condensed ureas are no longer used in IUPAC preferred names.
Examples:
N1-methyl-2-imido-1-thiodicarbonic diamide (PIN)
1-methyl-2-thiobiuret
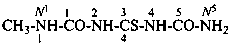
N1-methyl-2,4-diimido-3-thiotricarbonic diamide (PIN)
1-methyl-4-thiotriuret
Example:
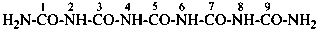
The traditional name ‘cyanamide’ is retained for NC-NH2 and is the preferred IUPAC name. Substitution is allowed on the –NH2 group. The systematic functional replacement name is carbononitridic amide.
Examples:
NC-N(CH2-CH3)2
diethylcyanamide (PIN)
diethylcarbononitridic amide
Systematic names for amides of the polycarbonic acids are formed by adding the functional class name ‘amide’ to that of the corresponding acid, preceded by the numerical prefix ‘di’ to indicate the presence of two –NH2 groups. Chalcogen analogues are described by functional replacement prefixes. Numerical and letter locants are used to number the structures.
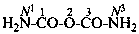
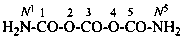
tricarbonic diamide (PIN)
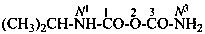
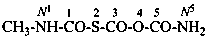
N1-methyl-2-thiotricarbonic diamide (PIN)
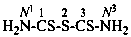
1,2,3-trithiodicarbonic diamide (PIN)
(not ‘thiuram monosulfide’)
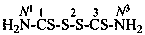
2-dithioperoxy-1,3-dithiodicarbonic diamide (PIN)
(not ‘thiuram disulfide’)
Amides follow acids, anhydrides, esters, and acid halides in the seniority order of compound classes expressed by suffixes (see P-41); within the amide class, amides rank in the same order as the corresponding acid. Seniority for numbering polyfunctional amides follows that described for acids, for which see P-65.1.2.3 and P-65.1.2.4.
Examples:
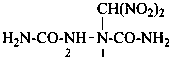
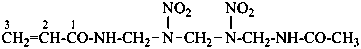
N-[({[(acetamidomethyl)nitramido]methyl}nitramido)methyl]prop-2-enamide (PIN)
N-{[{[(acetamidomethyl)(nitro)amino]methyl}(nitro)amino]methyl}prop-2-enamide
N-{[({[(acetylamino)methyl](nitro)amino}methyl)(nitro)amino]methyl}prop-2-enamide
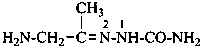
2-(1-aminopropan-2-ylidene)hydrazine-1-carboxamide (PIN)
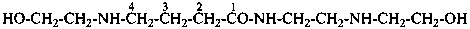
4-[(2-hydroxyethyl)amino]-N-{2-[(2-hydroxyethyl)amino]ethyl}butanamide (PIN)
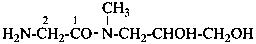
2-amino-N-(2,3-dihydroxypropyl)-N-methylacetamide (PIN)
P-66.2.1 Imides are compounds containing the structural grouping –CO-NH-CO–. Acyclic imides are N-acyl derivatives of primary amides and are named as such (see P-66.1.2.1). Cyclic imides are preferably named as heterocyclic pseudoketones. They may also be named by replacing the suffixes ‘dioic acid’, or ‘dicarboxylic acid’ of the corresponding dibasic acid, or ‘ic acid’ in retained names of diacids, by ‘imide’ or ‘dicarboximide’.
Examples:

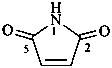
1H-pyrrole-2,5-dione (PIN)
pyrrole-2,5-dione
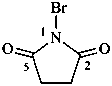
1-bromopyrrolidine-2,5-dione (PIN)
(not N-bromosuccinimide;
substitution is not allowed on succinimide)
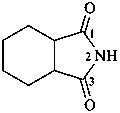
hexahydro-1H-isoindole-1,3(2H)-dione (PIN)
hexahydro-2H-isoindole-1,3-dione
cyclohexane-1,2-dicarboximide
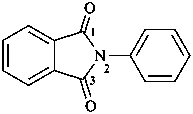
2-phenyl-1H-isoindole-1,3(2H)-dione (PIN)
2-phenyl-2H-isoindole-1,3-dione
N-phenylphthalimide
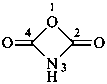
1,3-oxazetidine-2,4-dione (PIN)
P-66.3.0 DefinitionP-66.3.0 Definition
P-66.3.1 Systematic names
P-66.3.2 Substituent groups derived from hydrazides
P-66.3.3 Substituted hydrazides
P-66.3.4 Chalogen analogues of hydrazides
P-66.3.5 Hydrazides from carbonic, cyanic, and di- and polycarbonic acids
P-66.3.6 Semioxamazones
Hydrazides are compounds derived from the organic oxoacids denoted by a suffix, such as –COOH, –SO2-OH, –SO-OH, etc., by replacing –OH groups with –NH-NH2 groups.
P-66.3.1 Systematic names
Hydrazides of the type R-CO-NH-NH2 are named in two ways:
(1) by substitutive nomenclature;P-66.3.1.1 Substitutive nomenclature(2) by modification of retained names of carboxylic acids.
Hydrazides are named substitutively by using the following suffixes (see Table 4.4). The method of naming hydrazides as acyl derivatives of hydrazine is no longer recommended.
| –(C)O-NH-NH2 | hydrazide (preferred suffix) | |
| –CO-NH-NH2 | carbohydrazide (preferred suffix) | |
| –SO2-NH-NH2 | sulfonohydrazide (and corresponding Se and Te analogues; preselected suffixes) | |
| –SO-NH-NH2 | sulfinohydrazide (and corresponding Se and Te analogues; preselected suffixes) |
For naming acyclic hydrazides, the suffix ‘hydrazide’ is recommended in place of ‘ohydrazide’ in accordance with the general use of suffixes added to names of parent hydrides, for example pentanehydrazide for CH3-CH2-CH2-CH2-CO-NH-NH2, not pentanohydrazide.
The suffix ‘hydrazide’ is used to name acyclic compounds. The suffix ‘carbohydrazide’ is used to denote the –CO-NH-NH2 characteristic group attached to cyclic compounds and in chains having more than two –CO-NH-NH2 characteristic groups, or when the group is attached to a heteroatom of a heterocycle or parent compound. Multiplicative nomenclature may be used when the symmetry conditions for its use are met.
Nitrogen atoms in hydrazides are identified by the locants N and N′ as 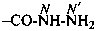 , even though hydrazine itself, is numbered using the numerical locants 1 and 2,
, even though hydrazine itself, is numbered using the numerical locants 1 and 2, 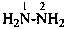 . When two hydrazide suffixes are attached to an acyclic alkane, the suffix in position 1 is labeled N1, N′1, a second suffix in position ‘x’, Nx, N′x (see P-16.9).
. When two hydrazide suffixes are attached to an acyclic alkane, the suffix in position 1 is labeled N1, N′1, a second suffix in position ‘x’, Nx, N′x (see P-16.9).
Examples:
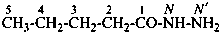
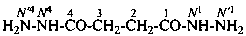
butanedihydrazide (PIN)
succinohydrazide (see P-66.3.1.2)
[not (ethane-1,2-diyldicarbonyl)dihydrazine]
[not succinyldihydrazine]
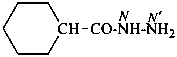
cyclohexanecarbohydrazide (PIN)
[not (cyclohexanecarbonyl)hydrazine]
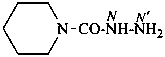
piperidine-1-carbohydrazide (PIN)
[not (piperidine-1-carbonyl)hydrazine]
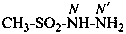
methanesulfonohydrazide (PIN)
[not (methanesulfonyl)hydrazine]
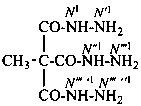
ethane-1,1,1-tricarbohydrazide (PIN)
Names of hydrazides are formed by changing the ‘ic acid’ or ‘-oic acid’ ending of the retained names of carboxylic acids into ‘ohydrazide’ (for nitrogen locants, see P-66.3.3).
P-66.3.1.2.1 Only the following five names are preferred IUPAC names and can be substituted in the same way as corresponding amides (see P-66.1.1.1.2). Systematic substitutive names are used to generate acids modified by functional replacement.
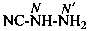
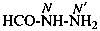
formohydrazide (PIN)
(not hydrazinecarbaldehyde; see P-66.6.1.3)
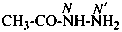
acetohydrazide (PIN)
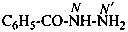
benzohydrazide (PIN)
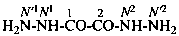
oxalohydrazide (PIN)
Example:
Examples:
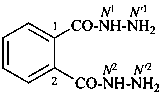
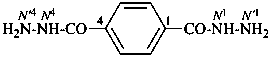
benzene-1,4-dicarbohydrazide (PIN)
terephthalohydrazide
Example:
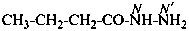
Examples:
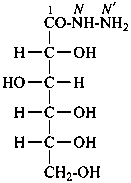
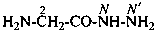
2-aminoacetohydrazide
glycinohydrazide
Substituent groups corresponding to hydrazides are of two types: –CO-NH-NH2 , –SO2-NH-NH2, etc.; and –NH-NH-CO-R , –NH-NH-SO2-R , etc.
P-66.3.2.1 Substituent groups of the type –CO-NH-NH2, –SO2-NH-NH2, etc., may be named in three ways except when the –CO-NH-NH2 group is at the end of a carbon chain:
(1) as an acyl group derived from the corresponding acid, which gives the preferred prefix; orExamples:(2) as the appropriate carbonohydrazidoyl acyl prefix; or
(3) by concatenation using the prefix hydrazinyl with carbonyl.
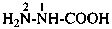
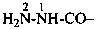
(1) hydrazinecarbonyl (preferred prefix)
(2) carbonohydrazidoyl (see P-65.2.1.4)
(3) hydrazinylcarbonyl
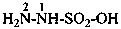
hydrazinesulfonic acid (preselected name)
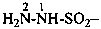
(1) hydrazinesulfonyl (preselecteded prefix, see P-65.3.2.2.2)
(3) hydrazinylsulfonyl
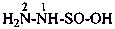
hydrazinesulfinic acid (preselected name)
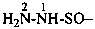
(1) hydrazinesulfinyl (preselecteded prefix: see P-65.3.2.2.2)
(3) hydrazinylsulfinyl
Examples:
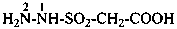
3-(hydrazinesulfinyl)naphthalene-2-carboxylic acid (PIN)
3-(hydrazinylsulfinyl)naphthalene-2-carboxylic acid
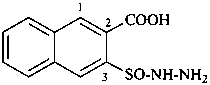
2-(hydrazinecarbonyl)benzene-1-sulfonic acid (PIN)
2-carbonohydrazidoylbenzene-1-sulfonic acid
Example:
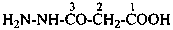
(1) by expressing the corresponding hydrazide as a prefix by replacing the final letter ‘e’ in the name of the hydrazide by the letter ‘o’, for example, ‘acetohydrazido’, ‘propanehydrazido’, and ‘benzohydrazido’; the locant N designates the nitrogen atom adjacent to the –CO– group.Method (1) generates preferred IUPAC names.(2) as an acylhydrazinyl substituent group; the hydrazinyl group is numbered by using numerical locants ‘1’ and ‘2’, the locant ‘1’ being the nitrogen atom adjacent to the free valence.
Examples:
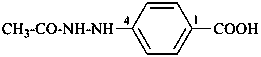
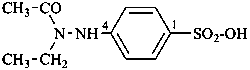
(1) 4-(N-ethylacetohydrazido)benzene-1-sulfonic acid (PIN)
(2) 4-(2-acetyl-2-ethylhydrazin-1-yl)benzene-1-sulfonic acid
Example:
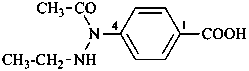
P-66.3.3.1 Alkyl, aryl, cycloalkyl, etc. substituents on the nitrogen atoms of hydrazides are described by the appropriate prefix names and the locants ‘N’ for –NH– and ‘N′’ for –NH2, as illustrated below. The locants ‘1′’and ‘2′’ have been used in the past for naming hydrazides as derivatives of hydrazine; these locants are no longer recommended. Preferred IUPAC names are hydrazide names and use the locants ‘N’ and ‘N′’
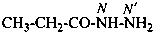
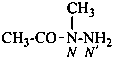
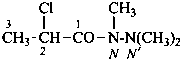
2-chloro-N,N′,N′-trimethylpropanehydrazide (PIN)
[not 1-(2-chloropropanoyl)-1,2,2-trimethylhydrazine]
Examples:
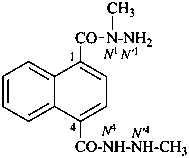
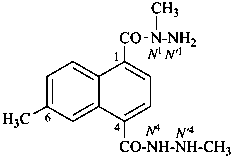
N1,N′4,6-trimethylnaphthalene-1,4-dicarbohydrazide (PIN)
(the numbering is based on the lowest set of locants for the three substituent groups,
and ‘N1,N′4,6’ is lower than ‘N′1,N4,7’)
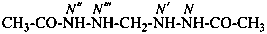
N′,N′′′-methylenediacetohydrazide (PIN, a multiplicative name)
Examples:
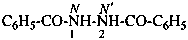
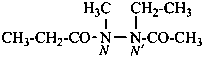
N′-acetyl-N′-ethyl-N-methylpropanehydrazide (PIN)
(not 1-acetyl-1-ethyl-2-methyl-2-propanoylhydrazine)
Chalcogen analogues of hydrazides are named substitutively using suffixes formed by functional replacement, i.e., ‘thiohydrazide’, ‘carbothiohydrazide’, ‘sulfonothiohydrazide’, etc., as described in P-33.2.2 and Table 4.4.
The following methods are no longer recommended:
(a) substitution of hydrazine with appropriately modified acyl groups;Examples:(b) modification of retained names by the prefixes ‘thio’, ‘seleno’, ‘telluro’.
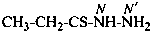
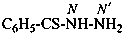
benzenecarbothiohydrazide (PIN)
[not (benzenecarbothioyl)hydrazine;
nor (thiobenzoyl)hydrazine]
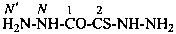
2-hydrazinyl-2-sulfanylideneacetohydrazide (PIN)
(not thiooxalic dihydrazide)
P-66.3.5.1 Preferred IUPAC names for hydrazides derived from carbonic and cyanic acids are chosen according to the seniority order of classes.
Examples:
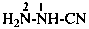
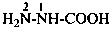
hydrazinecarboxylic acid (PIN)
carbonohydrazidic acid
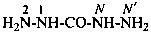
hydrazinecarbohydrazide (PIN)
carbonic dihydrazide
Examples:
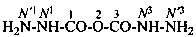
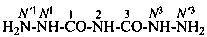
2-imidodicarbonic dihydrazide (PIN)
[(hydrazinecarbonyl)amino]formohydrazide
When a group having priority for citation as a principal characteristic group is present, the hydrazide group is named:
(1) as an acylhydrazinyl compound; the hydrazinyl group is numbered by the numerical locants 1 and 2;Method (2) generates preferred IUPAC names.(2) by expressing the corresponding hydrazide substitutively as a prefix by replacing the final letter ‘e’ in the name of the hydrazide by the letter ‘o’.
Examples:
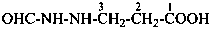
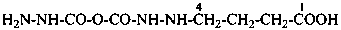
4-{[(hydrazinecarbonyl)oxy]formohydrazido}butanoic acid (PIN)
4-(2-{[(hydrazinecarbonyl)oxy]carbonyl}hydrazin-1-yl)butanoic acid
H2N-NH-CO-NH-NH-CH2-COOH
(hydrazinecarbohydrazido)acetic acid (PIN)
[2-(hydrazinecarbonyl)hydrazin-1-yl]acetic acid
[2-(hydrazinylcarbonyl)hydrazin-1-yl]acetic acid
Semioxamazones have the general structure R=N-NH-CO-CO-NH2. They are derivatives of the hydrazide of oxamic acid. Their preferred IUPAC names are based on the parent name acetamide, but they may also be named as derivatives of oxamic hydrazide.
Example:
P-66.4.1 AmidinesP-66.4.1 Amidines
P-66.4.2 Amidrazones
P-66.4.3 Hydrazidines
P-66.4.4 Amidoximes (amide oximes)
Compounds having the general structure R-C(=NH)-NH2 generically are known as ‘carboxamidines’ and those having the general structure R-S(=NH)-NH2 as ‘sulfinamidines’. Compounds having the structures below are known only as ‘sulfonimidamides’, and not as amidines.
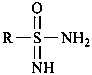 or
or 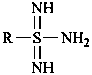
P-66.4.1.1 Suffixes for amidinesP-66.4.1.1 Suffixes for amidines
P-66.4.1.2 Amidines of carbonic, and di- and polycarbonic acids
P-66.4.1.3 Prefixes for the amidine characteristic group
P-66.4.1.4 Substituted amidines
P-66.4.1.5 Formamidine disulfides
P-66.4.1.6 Diamidides
Amidines are named as amides by functional replacement nomenclature in which the =O atom has been replaced by the =NH group. As a principal characteristic group they are designated by the suffixes ‘-imidamide’ and ‘-carboximidamide’. The locant for the –NH2 group is N and for the imino group N′. The suffixes ‘-amidine’ and ‘-carboxamidine’ are no longer recommended.
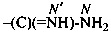
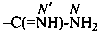
-carboximidamide (preferred suffix)
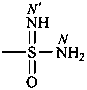
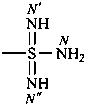
-sulfonodiimidamide (preselected suffix)
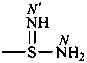
-sulfinimidamide (preselected suffix)
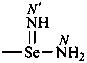
-seleninimidamide (preselected suffix)
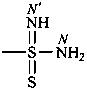
Retained names of amidines are formed by replacing the ‘amide’ ending in names of amides by ‘imidamide’, but these are not preferred IUPAC names; preferred IUPAC names are derived systematically. Other than that, the nomenclatural properties of amides are transferred to amidines; thus, names of amidines correspond to preferred names of amides. Amide names that are not substitutable generate nonsubstitutable amidine names.
Examples:
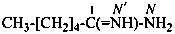
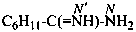
cyclohexanecarboximidamide (PIN)
(no longer cyclohexanecarboxamidine)
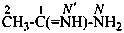
ethanimidamide (PIN)
acetimidamide
(no longer acetamidine)
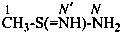
methanesulfinimidamide (PIN)
(no longer methanesulfinamidine)
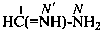
methanimidamide (PIN)
formimidamide
(no longer formamidine)
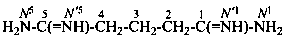
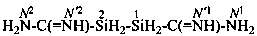
disilane-1,2-dicarboximidamide (PIN)
(no longer disilane-1,2-dicarboxamidine)
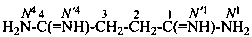
butanediimidamide (PIN)
succinimidamide
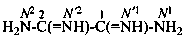
ethanediimidamide (PIN)
oxalimidamide
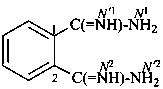
benzene-1,2-dicarboximidamide (PIN)
phthalimidamide
(no longer benzene-1,2-dicarboxamidine)
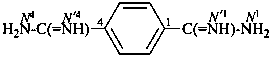
benzene-1,4-dicarboximidamide (PIN)
terephthalimidamide
(no longer benzene-1,4-dicarboxamidine)
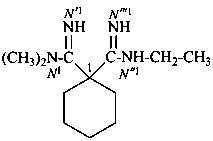
N′′1-ethyl-N1,N1-dimethylcyclohexane-1,1-dicarboximidamide (PIN)
P-66.4.1.2.1 Guanidine and its derivatives
P-66.4.1.2.1.1 The preferred IUPAC name for the ‘amidine’ related to carbonic acid, H2N-C(=NH)-NH2, is the retained name ‘guanidine’; the locants N, N′ and N′′ are used in preferred IUPAC names. The locants 1, 2, and 3 have been used but are no longer recommended even for general nomenclature.
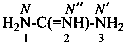
P-66.4.1.2.1.2 Hydrocarbyl derivatives are named as substituted guanidines. When the position of the double bond is unknown, the preferred IUPAC name uses a minimum number of primes.
Examples:
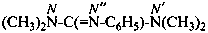
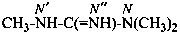
N,N,N′-trimethylguanidine (PIN)
N,N,N′-trimethylcarbonimidic diamide
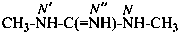 or
or 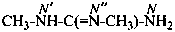
N,N′-dimethylguanidine (PIN)
(not N,N′′-dimethylguanidine)
N,N′-dimethylcarbonimidic diamide
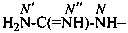
(H2N)2C=N–
(diaminomethylidene)amino (preferred prefix)
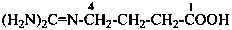
4-[(diaminomethylidene)amino]butanoic acid (PIN)
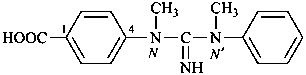
4-[methyl(N-methyl-N-phenylcarbamimidoyl)amino]benzoic acid (PIN)
4-({imino[methyl(phenyl)amino]methyl}methylamino)benzoic acid
4-(N,N′-dimethyl-N′-phenylcarbamimidamido)benzoic acid
H2N-C(=NH)-NH-CHO
N-carbamimidoylformamide (PIN)
N-[amino(imino)methyl]formamide
(not N-formylguanidine)
H2N-C(=NH)-NH-CO-NH2
N-carbamimidoylurea (PIN)
N-[amino(imino)methyl]urea
(not N-carbamoylguanidine)
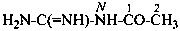
N-carbamimidoylacetamide (PIN)
N-(C-aminocarbonimidoyl)acetamide
N-[amino(imino)methyl]acetamide
(not N-acetylguanidine)
The names biguanide, triguanide, etc., are no longer recommended. Condensed guanidines, H2N-[C(=NH)-NH]n-H where n = 2, 3, or 4 are named systematically as the diamides of imidodicarbonimidic acid, diimidotricarbonimidic acid, and triimidotetracarbonimidic acid. Locants, as shown, are used to indicate the positions of substituents.
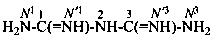
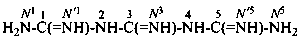
diimidotricarbonimidic diamide (PIN)
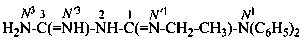
Example:
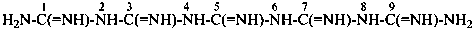
P-66.4.1.3.1 The systematic name for the group –C(=NH)-NH2 is ‘carbamimidoyl’; it is the name of the acyl group derived from the name carbamimidic acid, HO-C(=NH)-NH2 and is the preferred IUPAC prefix. The prefix ‘amidino’ is no longer recommended. In the acyl group, the –NH2 group is denoted by the locant N and the =NH group by N′.
The prefix ‘amidino’ is no longer acceptable in IUPAC nomenclature; ‘carbamimidoyl’ is now recommended for preferred IUPAC names.
Examples:
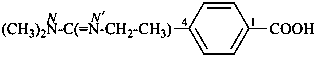
4-(N′-ethyl-N,N-dimethylcarbamimidoyl)benzoic acid (PIN)
4-[(dimethylamino)(ethylimino)methyl]benzoic acid
[not 4-(N′-ethyl-N,N-dimethylamidino)benzoic acid]
Example:
Example:
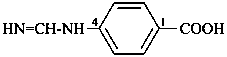
–S(=NH)2-NH2
S-aminosulfonodiimidoyl (preselected prefix)
–S(=NH)-NH2
S-aminosulfinimidoyl (preselected prefix)
Example:
(1) substitutively, by using a prefix formed by changing the final letter ‘e’ in the complete name of the amide to ‘o’, thus changing the suffixes ‘imidamide’ and ‘carboximidamide’ into ‘imidamido’ and ‘carboximidamido’, respectively, or ‘sulfonimidamide’ to ‘sulfonimidamido’, etc.Method (1) generates preferred IUPAC prefixes.(2) substitutively, by using ‘acylamino’ prefixes formed by substituting the name of the acyl group to the substituent ‘amino’
Examples:
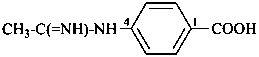
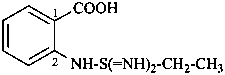
2-(ethanesulfonodiimidamido)benzoic acid (PIN)
2-(ethanesulfonodiimidoylamino)benzoic acid
P-66.4.1.4.1 N-Substituted amidines are named by prefixing the name of the appropriate substituent to the name of the unsubstituted imidamides, with N and N′ as locants when there is only one imidamide suffix; the locant N refers to the amino group and N′ refers to the imino group. The locants N and N′ with superscripted numbers for example, N1 or N′2, are used when there are two or more imidamide suffixes. Because of tautomerism, the locants N, N′, etc. are used when only one substituent is present on each imidamide group.
The locants N and N′ are used for the NH2 and NH group of amidines, respectively, rather than the locants N1 and N2 which were used in the 1979 recommendations (ref. 1).
Examples:
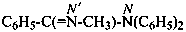
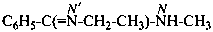
N′-ethyl-N-methylbenzenecarboximidamide (PIN)
(not N′-ethyl-N-methylbenzenecarboxamidine)
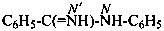
N-phenylbenzenecarboximidamide (PIN)
(not N-phenylbenzenecarboxamidine;
not benzimidanilide)
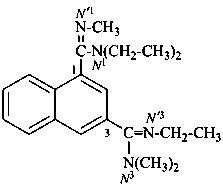
N1,N1,N′3-triethyl-N′1,N3,N3-trimethylnaphthalene-1,3-dicarboximidamide (PIN)
When geminal carboxamidine groups are present, the locants N, N′, N′′, N′′′ are used. Lowest locants are assigned to the most substituted group; when there is a choice, lowest locants are assigned to the first cited N-substituent. For polyamidines with at least one pair of geminal amidine groups, superscripted numerical locants indicating the position of the amidine group on a chain or cycle are used. This system of N-locants in association with superscript numerical locants is also recommended for naming disubstituted amines (see P-62.2.4.1) and disubstituted amides (see P-66.1.1.3.1.2).
Examples:
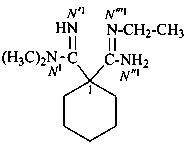
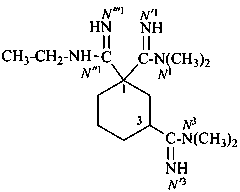
N′′1-ethyl-N1,N1,N3,N3-tetramethylcyclohexane-1,1,3-tricarboximidamide (PIN)
The compound H2N-C(=NH)-S-S-C(=NH)-NH2 and its derivatives have been named previously on the basis of the parent structure ‘formamidine disulfide’. They are now named on the basis of the parent compound ‘dicarbonic acid’ or as a dithioperoxyanhydride which is the preferred IUPAC name.
Example:
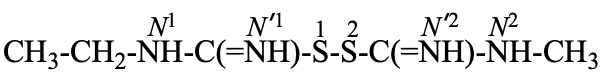
Note: The anhydride is the higher class in seniority (see P-41).
Diamidides are analogues of acyclic carboxylic anhydrides in which the =O atoms have been replaced by =NR groups and the anhydride oxygen atom by –NR– giving the general formula R-C(=NR′)-N(R′′)-C(=NR′′′)-R′′′ ′. Preferred IUPAC names are formed systematically as N-imidoylimidamides.
Example:
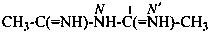
P-66.4.2.1 Amidrazone suffixes
Compounds having the general structure R-C(NH2)=N-NH2 or the tautomeric structure R-C(=NH)-NH-NH2 have the class name ‘amidrazones’ and are named substitutively using the suffixes ‘hydrazonamide’ or ‘carbohydrazonamide’, and ‘imidohydrazide’ or ‘carboximidohydrazide’, respectively. N-Substitution, when the position of the double bond is known, is designated with the following locants:
| for carbohydrazonamides | 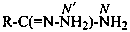 | |
| for carboximidohydrazides | 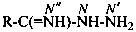 |
When the position of the double bond is unknown, the senior characteristic group, carbohydrazonamide, is chosen to denote the tautomeric structure, with appropriate locants N and N′ to denote substitution. When the position of the substituted suffixes must be indicated in a name, locants designating the positions of the suffixes are superscripted to the appropriate N locants.
Retained names of amidrazones are formed by replacing the ‘amide’ ending in names of amides by ‘ohydrazonamide’ and are only used in general nomenclature. Preferred IUPAC names of amidrazones are formed systematically. Otherwise, the nomenclatural properties of amides are transferred to amidrazones; thus, preferred names of amidrazones correspond to preferred names of amides; and amides that are not substitutable generate nonsubstitutable amidrazones.
Examples:
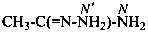
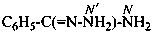
benzenecarbohydrazonamide (PIN)
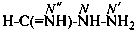
methanimidohydrazide (PIN)
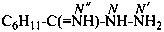
cyclohexanecarboximidohydrazide (PIN)
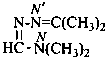
N,N-dimethyl-N′-(propan-2-ylidene)methanehydrazonamide (PIN)
N,N-dimethyl-N′-isopropylideneformohydrazonamide
(not N,N-dimethylformamide isopropylidenehydrazone)
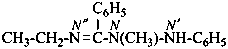
N′′-ethyl-N-methyl-N′-phenylbenzenecarboximidohydrazide (PIN)
N′′-ethyl-N-methyl-N′-phenylbenzimidohydrazide
(not N1-methyl-N2-phenylbenzohydrazide ethylimide)
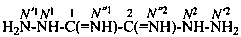
ethanediimidohydrazide (PIN)
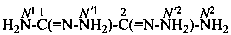
ethanedihydrazonamide (PIN)
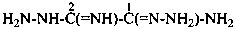
2-hydrazinyl-2-iminoethanehydrazonamide (PIN)
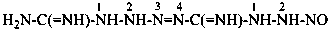
4-(2-nitrosohydrazine-1-carboximidoyl)tetraaz-3-ene-1-carboximidamide (PIN)
[an imidamide (an amidine) is senior to an imidohydrazide (an amidrazone)]
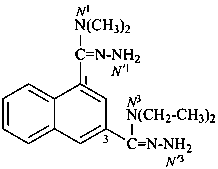
N3,N3-diethyl-N1,N1-dimethylnaphthalene-1,3-dicarbohydrazonamide (PIN)
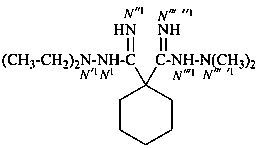
N′1,N′1-diethyl-N′′′ ′1,N′′′ ′1-dimethylcyclohexane-1,1-dicarboximidohydrazide (PIN)
Note: For locants N, N′, etc. in association with numerical locants, see P-16.9, P-62.2.4.1.2 and P-66.4.1.4.2)
Example:
The general methodology discussed in P-65.2 is applied to generate the names for amidrazones derived from carbonic, di-, and polycarbonic acids.
Examples:
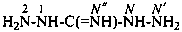
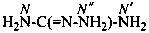
carbonohydrazonic diamide (PIN)
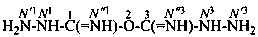
dicarbonimidic dihydrazide (PIN; see P-65.2.3.1)
(not 1,3-diimidodicarbonic dihydrazide)
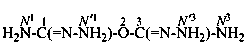
dicarbonohydrazonic diamide (PIN; see P-65.2.3.1)
(not 1,3-dihydrazonodicarbonic diamide)
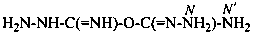
[(hydrazinecarboximidoyl)oxy]methanehydrazonamide (PIN)
[(carbamohydrazonoyl)oxy]methanimidohydrazide
[not [(hydrazinecarboximidoyl)oxy]formohydrazonamide]
P-66.4.2.3.1 Prefixes for the group –C(=NH)-NHNH2 are ‘hydrazinecarboximidoyl’ (preferred prefix) derived from hydrazinecarboximidic acid, and ‘carbonohydrazidimidoyl’, derived from carbonohydrazidimidic acid. When this group is located at the end of a carbon chain, the prefixes ‘imino’ and ‘hydrazinyl’ are used in preferred IUPAC names in order to avoid fragmenting the parent chain.
Examples:
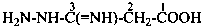
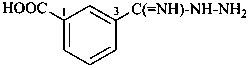
3-(hydrazinecarboximidoyl)benzoic acid (PIN)
3-carbonohydrazidimidoylbenzoic acid
3-[hydrazinyl(imino)methyl]benzoic acid
3-(C-hydrazinylcarbonimidoyl)benzoic acid
Examples:
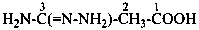
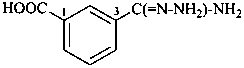
3-carbamohydrazonoylbenzoic acid (PIN)
3-[amino(hydrazinylidene)methyl]benzoic acid
Examples:
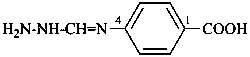
H2N-N=CH-NH-CH2-CH2-CN
N-(2-cyanoethyl)methanehydrazonamide (PIN)
(not 3-[(methanehydrazonoyl)amino]propanenitrile;
nor 3-[(hydrazinylidenemethyl)amino]propanenitrile)
Example:
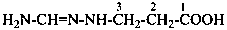
(1) substitutively, by using a prefix formed by changing the final letter ‘e’ in the complete name of the amide to ‘o’;Method (1) generates preferred prefixes.(2) substitutively, by using ‘acylamino’ prefixes formed by substituting the name of the acyl group to the substituent ‘amino’
Example:
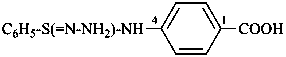
4-(benzenesulfinohydrazonamido)benzoic acid (PIN)
4-[(benzenesulfinohydrazonoyl)amino]benzoic acid
(1) by expressing the corresponding hydrazide as a prefix by replacing the final letter ‘e’ in the name of the hydrazide by the letter ‘o’:Method (1) generates preferred prefixes.(2) as an acylhydrazinyl prefix; the hydrazinyl group is numbered by using the numerical locants 1 and 2.
Examples:
CH3-C(=NH)-NH-NH-CH2-CO-O-CH3
methyl (ethanimidohydrazido)acetate (PIN)
methyl [2-(ethanimidoyl)hydrazin-1-yl]acetate
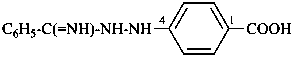
(1) 4-(benzenecarboximidohydrazido)benzoic acid (PIN)
(2) 4-[2-(benzenecarboximidoyl)hydrazin-1-yl]benzoic acid
P-66.4.3.1 Hydrazidine suffixes
Compounds with the general structure R-C(=N-NH2)-NH-NH2 have the class name ‘hydrazidines’ and are named substitutively by using the suffixes ‘hydrazonohydrazide’ and ‘carbohydrazonohydrazide’ as prescribed for hydrazides. The former method of naming hydrazidines as hydrazones of the corresponding hydrazides (see C-954.2 in ref. 1) is no longer recommended.
Hydrazidines are named systematically in these recommendations rather than as hydrazones of the corresponding hydrazides as in the 1979 recommendations.
Locants are assigned to nitrogen atoms as follows:
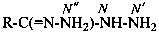
Names of hydrazidines formally derived from carboxylic acids having retained names, formed by replacing the ‘ohydrazide’ ending in names of hydrazides by ‘hydrazonohydrazide’, may be used in general nomenclature. Preferred IUPAC names are formed systematically. Otherwise, the nomenclatural properties of hydrazides are transferred to hydrazidines; thus, preferred names of hydrazidines correspond to preferred names of hydrazides, and hydrazides that are not substitutable generate nonsubstitutable hydrazidines.
Examples:
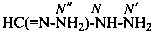
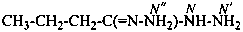
butanehydrazonohydrazide (PIN)
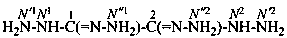
ethanedihydrazonohydrazide (PIN)
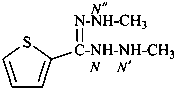
N′,N′′-dimethylthiophene-2-carbohydrazonohydrazide (PIN)
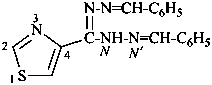
N′,N′′-dibenzylidene-1,3-thiazole-4-carbohydrazonohydrazide (PIN)
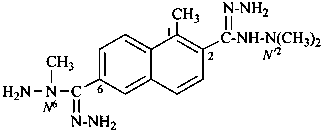
N′2, N′2, N6,1-tetramethylnaphthalene-2,6-dicarbohydrazonohydrazide (PIN)
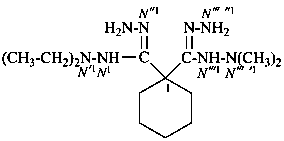
N′1,N′1-diethyl-N′′′ ′1,N′′′ ′1-dimethylcyclohexane-1,1-dicarbohydrazonohydrazide (PIN)
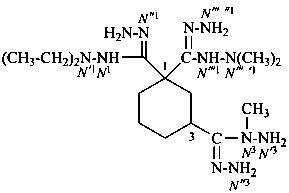
N′1, N′1-diethyl-N′′′ ′1,N′′′ ′1,N 3-trimethylcyclohexane-1,1,3-tricarbohydrazonohydrazide (PIN)
(for locants N, N′, etc. in association with numerical locants,
see P-16.9, P-62.2.4.1.2, P-66.3.1.2, P-66.3.3, P-66.4.1.1, P-66.4.1.4, and P-66.4.2.1)
Example:
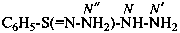
Examples:
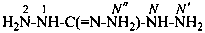
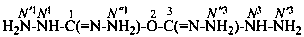
dicarbonohydrazonic dihydrazide (PIN, P-65.2.1.3)
(not dihydrazonodicarbonic dihydrazide)
P-66.4.3.4.1 In the presence of a senior characteristic group, the prefixes for the group –C(=N-NH2)-NH-NH2 are ‘hydrazinecarbohydrazonoyl’ (preferred prefix) or ‘C-hydrazinylcarbonohydrazonoyl’. When this group is located at the end of a carbon chain, the prefixes ‘hydrazinyl’ and ‘hydrazinylidene’ are preferred in order to avoid fragmenting the chain.
Examples:
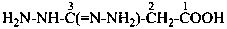
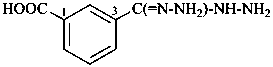
3-(hydrazinecarbohydrazonoyl)benzoic acid (PIN)
3-(C-hydrazinylcarbonohydrazonoyl)benzoic acid
3-[hydrazinyl(hydrazinylidene)methyl]benzoic acid
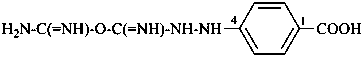
4-[C-(carbamimidoyloxy)methanimidohydrazido]benzoic acid (PIN)
4-{2-[C-(carbamimidoyloxy)methanimidoyl]hydrazin-1-yl}benzoic acid
Example:
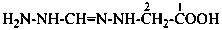
Amide oximes are formally oximes of carboxamides, i.e., compounds having the general structure R-C(=N-OH)-NH2 and derivatives formed by substitution. Preferred IUPAC names are N′-hydroxy or N′-(alkyloxy) derivatives of carboximidamides (amidines). Suffixes such as ‘amide oxime’ or ‘carboxamide oxime’ are no longer recommended.
Examples:
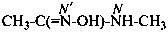
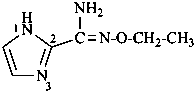
N′-ethoxy-1H-imidazole-2-carboximidamide (PIN)
(not imidazole-2-carboxamide O-ethyloxime)
P-66.5.0 IntroductionP-66.5.0 Introduction
P-66.5.1 Nomenclature for generating preferred names for nitriles
P-66.5.2 Substituted nitriles
P-66.5.3 Nitriles/cyanides corresponding to carbonic and di- and polycarbonic acids
P-66.5.4 Nitrile oxides and chalcogen analogues
Compounds with the general structure R-C≡N are called ‘nitriles’ or ‘cyanides’. Nitriles and cyanides are derived from hydrocyanic acid, H-C≡N. When the point of attachment of the –C≡N group to ‘R’ is a carbon atom or a heteroatom, these compounds form the class of nitriles and are named substitutively as nitriles. They may also be named as cyanides according to the principles of functional class nomenclature. In substitutive nomenclature formonitrile is the preferred IUPAC name. So, when substituted the compound is a preferred IUPAC name.
These two types of nomenclature are fully discussed in this Section.
P-66.5.1 Nomenclature for generating preferred names of nitriles
Compounds of the general structure R-C≡N have the class names ‘nitriles’ and are named in three ways:
(1) substitutively, using the suffixes ‘nitrile’ for –(C)N and ‘carbonitrile for –CN;P-66.5.1.1 Substitutive and functional class names for nitriles(2) by changing the ‘ic acid’ or ‘oic acid’ endings in retained names of carboxylic acids into ‘onitrile’; the nomenclatural properties of acids are transferred to nitriles; thus, preferred names of nitriles correspond to preferred names of carboxylic acids (see P-65.1.1.1) and carboxylic acids that are not substitutable generate nonsubstitutable nitriles (see P-65.1.1.2)
(3) by functional class nomenclature, using the class name ‘cyanide’
P-66.5.1.1.1 Acyclic mono- and dinitriles are named in the following two ways:
(1) substitutively by using the suffix ‘nitrile’; andMethod (1) leads to preferred IUPAC names.(2) by functional class nomenclature using the class name ‘cyanide’.
Examples:
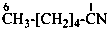

pentanedinitrile (PIN)
propane-1,3-diyl dicyanide
Example:
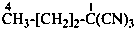
Examples:
H2N-NH-CN
hydrazinecarbonitrile
cyanohydrazide (PIN; see P-66.3.1.2.1)
hydrazinyl cyanide (see P-66.5.1.3)
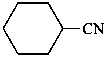
cyclohexanecarbonitrile (PIN)
cyclohexyl cyanide
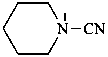
piperidine-1-carbonitrile (PIN)
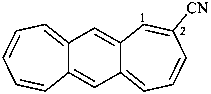
(benzo[1,2:4,5]di[7]annulene)-2-carbonitrile (PIN)
(benzo[1,2:4,5]dicycloheptene)-2-carbonitrile
Examples:
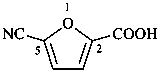
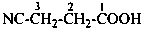
3-cyanopropanoic acid (PIN)
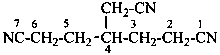
4-(cyanomethyl)heptanedinitrile (PIN)
P-66.5.1.2.1 The following names are preferred IUPAC names, with unlimited substitution, except for formonitrile whose substitution rules are the same as formic acid (see P-65.1.8) and, obviously, for oxalonitrile for which no substitution is possible.
CH3-CN
acetonitrile (PIN)
ethanenitrile
C6H5-CN
benzonitrile (PIN)
benzenecarbonitrile
NC-CN
oxalonitrile (PIN)
ethanedinitrile
Examples:
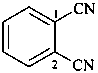
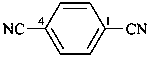
terephthalonitrile
benzene-1,4-dicarbonitrile (PIN)
Examples:
NC-CH2-CH2-CN
succinonitrile
butanedinitrile (PIN)
Examples:
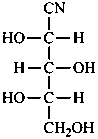
H2N-CH2-CN
aminoacetonitrile (see P-103.2.8)
glycinonitrile
Functional class nomenclature is used, when needed, to name compounds in accordance with the seniority of classes and to name compounds that cannot be named substitutively, for example, cyanides corresponding to sulfonic acid, sulfinic acids and their selenium and tellurium analogues, carbonic acid, cyanic acid, and inorganic acids.
P-66.5.1.3.1 Nitriles with an α-oxo group.
Compounds of the type R-CO-CN can be named as acyl cyanides in a way similar to acid halides. Since acyl cyanides are senior to nitriles, in the seniority of classes, functional class nomenclature must be used to express correctly the seniority order.
Examples:
CH3-CO-CN
acetyl cyanide (PIN)
2-oxopropanenitrile
CH3-[CH2]5-CO-CN
heptanoyl cyanide (PIN)
2-oxooctanenitrile
NC-CO-CO-CN
oxalyl dicyanide (PIN)
2,3-dioxobutanedinitrile
Examples:
C6H5-SeO-CN
benzeneseleninyl cyanide (PIN)
Substituents on the parent hydrides are denoted as prefixes. Nitriles, in the seniority order of classes, are senior to ketones, pseudoketones, heterones, hydroxy compounds, amines, and imines; these classes must be cited as prefixes in the presence of a nitrile group. Seniority for numbering of polyfunctional nitriles follows that described for acids, for which see P-65.1.2.3 and P-65.1.2.4.
Examples:
NC-CH2-CH2-NH-CH2-CH2-CN
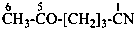
5-oxohexanenitrile (PIN)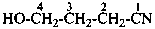
4-hydroxybutanenitrile (PIN)
(not 4-hydroxybutyronitrile;
no substitution for this retained name, see P-65.1.1.2)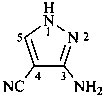
3-amino-1H-pyrazole-4-carbonitrile (PIN)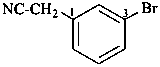
(3-bromophenyl)acetonitrile (PIN)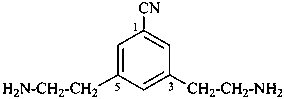
3,5-bis(2-aminoethyl)benzonitrile (PIN)
3,3′-azanediyldipropanenitrile (PIN)
(not 3,3′-azanediyldipropionitrile;
no substitution on propionitrile, see P-65.1.1.2)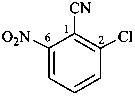
2-chloro-6-nitrobenzonitrile (PIN)
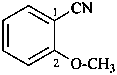
2-methoxybenzonitrile (PIN)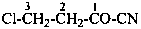
3-chloropropanoyl cyanide (PIN)
4-chloro-2-oxobutanenitrile
(not 3-chloropropionyl cyanide)
P-66.5.3.1 Nitriles corresponding to carbonic acid and di- and polycarbonic acids are named by functional class nomenclature.
Examples:
NC-CO-CO-CN
oxalyl dicyanide (PIN)
2,3-dioxobutanedinitrile
NC-C(=NH)-CN
carbonimidoyl dicyanide (PIN)
(not 2-iminopropanedinitrile)
NC-C(=NNH2)-CN
carbonohydrazonoyl dicyanide (PIN)
(not 2-hydrazonopropanedinitrile)
H2N-CO-CN
carbamoyl cyanide (PIN)
NC-CO-O-CO-CN
dicarbonic dicyanide (PIN) (see P-65.5.3.2)
P-66.5.4.1 Compounds with the general structure R-C≡NO have the generic name ‘nitrile oxides’. As they may be considered as zwitterions, they are classed with zwitterions in the order of compound classes. They are named by three methods:
(1) by the term ‘oxide’, ‘sulfide’, ‘selenide’, or ‘telluride’ added to the name of the nitrile (see P-74.2.2.2.1.2).Method (1) leads to preferred IUPAC names(2) by applying the λ-convention and oxo substitution to the nitrogen atom (see P-14.1);
(3) as zwitterions (see P-74.2.2.2.1.2).
Examples:
HC≡N+–O–
(1) formonitrile oxide (PIN)
(2) methylidyne(oxo)-λ5-azane
(3) (methylidyneazaniumyl)oxidanide
Examples:
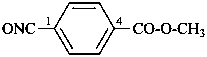
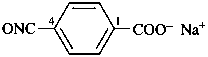
sodium 4-[(oxo-λ5-azanylidyne)methyl]benzoate (PIN)
(no longer sodium 4-isofulminatobenzoate)
P-66.6.0 IntroductionP-66.6.0 Introduction.
P-66.6.1 Systematic names of aldehydes
P-66.6.2 Aldehydes from di- and polycarbonic acids
P-66.6.3 Chalcogen analogues of aldehydes
P-66.6.4 Polyfunctional aldehydes
P-66.6.5 Acetals and ketals, hemiacetals and hemiketals, and their chalcogen analogues
The class name ‘aldehyde’ traditionally refers to compounds containing the –CH=O group attached to a carbon atom. However, nomenclature for aldehydes has been extended to describe a –CHO group attached to a heteroatom.
P-66.6.1 Systematic names of aldehydes
Aldehydes are systematically named in three ways:
(1) substitutively, using the suffixes ‘al’ for –(C)HO and ‘carbaldehyde’ for –CHO;P-66.6.1.1 Names based on suffixes(2) by changing the ‘ic acid’ or ‘oic acid’ endings of retained names of carboxylic acids into ‘aldehyde’; the nomenclatural properties of acids are transferred to aldehydes; thus, preferred names of aldehydes correspond to preferred names of acids, and carboxylic acids that are not substitutable generate nonsubstitutable aldehydes;
(3) by using the prefixes ‘oxo’, denoting =O, or ‘formyl-’, denoting the substituent group –CHO.
P-66.6.1.1.1 Mono- and dialdehydes derived from alkanes are named substitutively using the suffix ‘al’ added to the name of the parent hydride with elision of the final letter ‘e’ of the parent hydride before ‘a’.
Examples:
OHC-CH2-CH2-CH2-CHO
pentanedial (PIN)
Example:
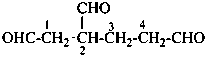
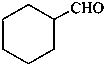
Examples:
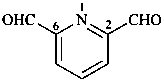
pyridine-2,6-dicarbaldehyde (PIN)
H2P-CHO
phosphanecarbaldehyde (PIN)
H2NNH-CHO
hydrazinecarbaldehyde
formohydrazide (PIN;
hydrazide is senior to an aldehyde)
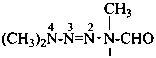
1,4,4-trimethyltetraaz-2-ene-1-carbaldehyde (PIN)
Names of aldehydes derived from retained names are formed by changing the ‘ic acid’ or ‘oic acid’ ending of the retained names of carboxylic acids to ‘aldehyde’. Substitution of aldehydes parallels that of corresponding carboxylic acids (see P-65.1.1.2).
P-66.6.1.2.1 The following names are preferred IUPAC names, with substitution allowed for acetaldehyde and benzaldehyde. Substitution rules for formaldehyde are the same as for formic acid (see P-65.1.8).
CH3-CHO
acetaldehyde (PIN)
ethanal
C6H5-CHO
benzaldehyde (PIN)
benzenecarbaldehyde
Examples:
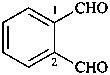
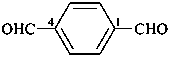
terephthalaldehyde
benzene-1,4-dicarbaldehyde (PIN)
Examples:
OHC-CH2-CH2-CHO
succinaldehyde
butanedial (PIN)
Examples:
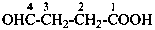
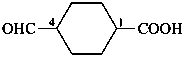
4-formylcyclohexane-1-carboxylic acid (PIN)
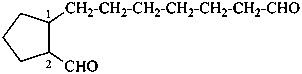
2-(7-oxoheptyl)cyclopentane-1-carbaldehyde (PIN)
7-(2-formylcyclopentyl)heptanal
(ring preferred to chain: see P-44.1.2.2)
Aldehydes from di- and polycarbonic acids are named on the basis of the higher compound class. Multiplicative names based on formaldehyde can be used (see P-15.3.2.1).
Examples:
O=CH-O-CO-O-CH=O
carbonic diformic dianhydride (PIN)
(anhydride senior to ester; see P-41)
bis(oxomethyl) carbonate
Chalcogen analogues of aldehydes are named by using the suffixes and prefixes in Table 6.4. In the seniority order of classes, aldehydes are senior to ketones, hydroxy compounds, amines, and imines. Names of chalcogen analogues corresponding to aldehydes with retained names are all systematically formed.
| Group | Suffix | Prefix | ||
| –(C)HS | thial | sulfanylidene (preferred prefix) thioxo | ||
| –(C)HSe | selenal | selanylidene (preferred prefix) selenoxo | ||
| –(C)HTe | tellanal | tellanylidene (preferred prefix) telluroxo | ||
| –CHS | carbothialdehyde | methanethioyl (preferred prefix) thioformyl | ||
| –CHSe | carboselenaldehyde | methaneselenoyl (preferred prefix) selenoformyl | ||
| –CHTe | carbotelluraldehyde | methanetelluroyl (preferred prefix) telluroformyl | ||
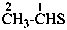
C6H5-CHS
benzenecarbothialdehyde (PIN)
thiobenzaldehyde
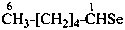
hexaneselenal (PIN)
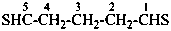
pentanedithial (PIN)
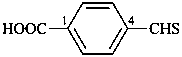
4-(methanethioyl)benzoic acid (PIN)
4-(thioformyl)benzoic acid
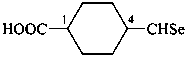
4-(methaneselenoyl)cyclohexane-1-carboxylic acid (PIN)
4-(selenoformyl)cyclohexane-1-carboxylic acid
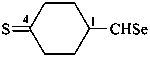
4-sulfanylidenecyclohexane-1-carboselenaldehyde (PIN)
4-thioxocyclohexane-1-carboselenaldehyde
In the presence of an aldehyde group, ketones, pseudoketones, heterones, hydroxy compounds, amines, and imines are expressed by prefixes. Seniority for numbering polyfunctional aldehydes follows that described for acids, for which see P-65.1.2.3 and P-65.1.2.4.
Examples:
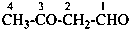
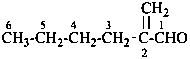
2-methylidenehexanal (PIN)
[not 2-butylprop-2-enal,
the longest chain is the principal chain (see P-44.3)]
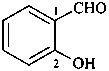
2-hydroxybenzaldehyde (PIN)
(not salicylaldehyde)
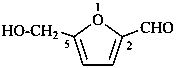
5-(hydroxymethyl)furan-2-carbaldehyde (PIN)
5-(hydroxymethyl)-2-furaldehyde
[not 5-(hydroxymethyl)furfural]
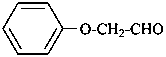
phenoxyacetaldehyde (PIN)
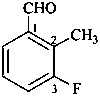
3-fluoro-2-methylbenzaldehyde (PIN)
P-66.6.5.1 Acetals and ketalsP-66.6.5.1 Acetals and ketals
P-66.6.5.2 Hemiacetals and hemiketals
P-66.6.5.3 Chalcogen analogues of acetals and ketals
P-66.6.5.1.1 Compounds with the general structure RR′C(O-R′′)(O-R′′′), where only R and R′ may be, but need not be, hydrogen, have the class name ‘acetal’. ‘Ketals’ constitute a subclass of acetals wherein neither R nor R′ may be hydrogen. Acetals (ketals) are named in two ways:
(1) substitutively as ‘alkoxy’, alkyloxy, ‘aryloxy’, etc. derivatives of an appropriate parent hydride or functional parent compound;Method (1), the substitutive method, leads to preferred IUPAC names.(2) by functional class nomenclature by citing the name of the aldehyde or ketone, the names of the O-substituents, in alphanumerical order if required, and finally the class terms ‘acetal’ or ‘ketal’.
Examples:
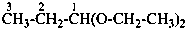
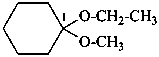
(1) 1-ethoxy-1-methoxycyclohexane (PIN)
(2) cyclohexanone ethyl methyl ketal
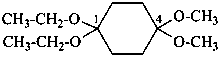
(1) 1,1-diethoxy-4,4-dimethoxycyclohexane (PIN)
(2) cyclohexane-1,4-dione 1,1-diethyl 4,4-dimethyl diketal
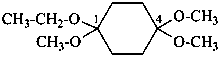
(1) 1-ethoxy-1,4,4-trimethoxycyclohexane (PIN)
(2) cyclohexane-1,4-dione 1-ethyl 1,4,4-trimethyl diketal
Cyclic acetals as the principal function are named as heterocyclic compounds and these names are preferred IUPAC names; cyclic ketals are spiro compounds that are named in accordance with the rules described in Section P-24 giving preferred IUPAC names.
Functional class nomenclature using the name of the appropriate divalent substituent groups may be used in general nomenclature.
Examples:
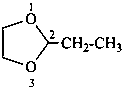
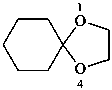
1,4-dioxaspiro[4.5]decane (PIN)
cyclohexanone ethylene ketal
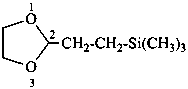
[2-(1,3-dioxolan-2-yl)ethyl]tri(methyl)silane (PIN)
3-(trimethylsilyl)propanal ethylene ketal
Compounds with the general structure RR′C(OH)(O-R′′) have the class name ‘hemiacetals’. They are named substitutively as ‘alkoxy’, ‘alkyloxy’, ‘aryloxy’, etc. derivatives of an appropriate hydroxy parent compound, such as an alcohol; these names are the preferred IUPAC names. Other names are formed by functional class nomenclature using the class name ‘hemiacetal’; similarly derivatives of ketones are denoted by the class name ‘hemiketal’.
Examples:
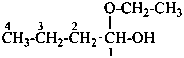
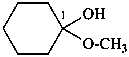
1-methoxycyclohexan-1-ol (PIN)
cyclohexanone methyl hemiketal
Sulfur analogues of acetals and ketals with the general structures RR′C(S-R′′)(S-R′′′), or RR′C(S-R′′)(O-R′′′), have the class names ‘dithioacetals’ or ‘monothioacetals’, respectively. They are named substitutively as ‘alkylsulfanyl’, ‘arylsulfanyl’, ‘alkoxy’, or ‘aryloxy’ derivatives, as appropriate, of a parent hydride; these names are preferred IUPAC names. Other names can be generated by functional class nomenclature, using class names such as ‘monothioacetal’ and ‘dithioketal’. Capital italic letter locants are used to provide structural specificity. Selenium, tellurium, and mixed chalcogen analogues are treated in the same way as their sulfur analogues.
Examples:
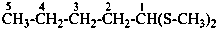
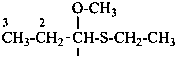
1-(ethylsulfanyl)-1-methoxypropane (PIN)
propanal S-ethyl O-methyl monothioacetal
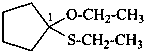
1-ethoxy-1-(ethylsulfanyl)cyclopentane (PIN)
cyclopentanone diethyl monothioketal
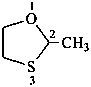
2-methyl-1,3-oxathiolane (PIN)
acetaldehyde ethylene monothioacetal
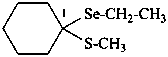
1-(ethylselanyl)-1-(methylsulfanyl)cyclohexane (PIN)
cyclohexanone Se-ethyl S-methyl selenothioketal
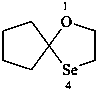
1-oxa-4-selenaspiro[4.4]nonane (PIN)
cyclopentanone ethylene monoselenoketal
Sulfur analogues of hemiacetals and hemiketals with the general structures RR′C(SH)(S-R′′), RR′C(OH)(S-R′′) or RR′C(SH)(O-R′′), have the class names ‘dithiohemiacetals’ or ‘monothiohemiacetals’, respectively. They are named substitutively as ‘alkylsulfanyl’, ‘arylsulfanyl’, ‘alkoxy’, or ‘aryloxy’ derivatives, as appropriate, of a hydroxy parent compound; these are preferred IUPAC names. Other names can be generated by functional class nomenclature. Capital italic letter locants are used to provide structural specificity. Selenium, tellurium, and mixed analogues are treated in the same way as their sulfur analogues; generically, they are ‘monoselenohemiacetals’, ‘ditellurohemiacetals’, ‘selenothiohemiacetals’, etc.
Examples:
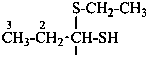
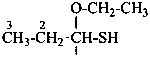
1-ethoxypropane-1-thiol (PIN)
propanal O-ethyl monothiohemiacetal
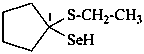
1-(ethylsulfanyl)cyclopentane-1-selenol (PIN)
cyclopentanone S-ethyl selenothiohemiketal
P-67.0 IntroductionP-67.0 INTRODUCTION
P-67.1 Mononuclear noncarbon oxoacids
P-67.2 Di- and polynuclear noncarbon oxoacids
P-67.3 Substitutive names and functional class names of polyacids
Mono-, di-, and polynuclear noncarbon oxoacids and their chalcogen analogues having retained names are used as parent structures to generate names of carbon containing compounds. In these recommendations, the names for these compounds are preselected names (see P-12.2)
Names of chalcogen analogues of noncarbon oxoacids are formed by functional replacement nomenclature. This type of nomenclature is also used to create derived classes, for example, acid halides and pseudohalides, amides, hydrazides, and amidines. With regard to functional replacement, mono- di- and polynuclear noncarbon acids do not constitute a homogeneous group. Names of mononuclear oxoacids are modified by infixes, with the exception of silicic acid, nitrous acid, nitric acid and the halogen acids. Names of the di- and polynuclear noncarbon oxoacids are modified by prefixes. Functional class nomenclature is used to generate names for esters, organic anhydrides, carbon containing pseudohalides such as cyanides and isocyanates, and organic derivatives of amides, imides, and hydrazides.
Mononuclear noncarbon oxoacids are discussed first, then di- and polynuclear noncarbon oxoacids, such as diphosphoric acid, (HO)2P(O)-O-P(O)(OH)2, which are named as acids, not as anhydrides, and hypodiphosphoric acid, (HO)2P(O)-P(O)(OH)2.
Systematization has been achieved, taking into consideration the nomenclature of inorganic compounds (ref. 12), that has restricted the use of retained names and of prefixes such as ‘hypo’, ‘ortho’, ‘iso’ added to the names of mononuclear oxoacids to generate retained names. However, the traditional nomenclature of organic compounds derived from mono-, di-, and polynuclear oxoacids has been maintained.
For carbonic, cyanic, di- and polynuclear carbonic acids, see P-65.2.
P-67.1 MONONUCLEAR NONCARBON OXOACIDS
Retained names for mononuclear noncarbon oxoacids have the following elements as central atoms: N, P, As, Sb, Si, B, S, Se, Te, F, Cl, Br, and I. They are used as parent structures and also for derivation of prefixes to be used in the presence of classes that have seniority for being named as parent compounds. These parent structures have retained names that are traditional names used as preselected names (see P-12.2). They may also have systematic additive or substitutive names, but these names are not recommended for generating preselected names (see IR-8, ref. 12)
Functional replacement nomenclature is discussed first; next the formation of esters and anhydrides using functional class nomenclature; and finally, substitutive nomenclature using prefixes is described. Application of the seniority order of oxoacids and their derivatives, described in Section P-42, is discussed. The Section ends with the nomenclature of aci-nitro compounds that are named as derivatives of azinic acid.
P-67.1.1 Names for mononuclear noncarbon oxoacids and their derivatives formed by substitutionP-67.1.1 Names for mononuclear noncarbon oxoacids and their derivatives formed by substitution
P-67.1.2 Functional replacement nomenclature applied to noncarbon oxoacids
P-67.1.3 Salts, esters, and anhydrides of noncarbon oxoacids
P-67.1.4 Substituent prefix groups derived from mononuclear noncarbon oxoacids
P-67.1.5 Seniority order among noncarbon oxoacids and derivatives
P-67.1.6 aci-Nitro compounds
P-67.1.1.1 Names of mononuclear noncarbon oxoacids
Preselected names (see P-12.2) of the mononuclear noncarbon oxoacids used for deriving preferred IUPAC names for organic compounds and names for general organic nomenclature are noted in the following list, given in alphabetical order.
| H2As(O)(OH) | arsinic acid (preselected name) | |
| H2As(OH) | arsinous acid (preselected name) | |
| HAs(O)(OH)2 | arsonic acid (preselected name) | |
| HAs(OH)2 | arsonous acid (preselected name) | |
| As(O)(OH)3 | arsoric acid (preselected name) arsenic acid | |
| Note: Arsoric acid is preferred to arsenic acid as the preselected name for clarity and consistency with phosphoric acid. | ||
| As(OH)3 | arsorous acid (preselected name) (formerly arsen(i)ous acid) | |
| Note: Arsorous acid is preferred to arsen(i)ous acid as the preselected name for clarity and consistency with phosphorous acid | ||
| H2N(O)(OH) | azinic acid (preselected name) | |
| H2N-OH | azinous acid hydroxylamine (preselected name, for which see P-68.3.1.1.1) | |
| HN(O)(OH)2 | azonic acid (preselected name) | |
| HN(OH)2 | azonous acid (preselected name) | |
| N(O)(OH)3 | nitroric acid (preselected name) | |
| N(OH)3 | azorous acid (preselected name) | |
| B(OH)3 | boric acid (preselected name) | |
| H2B(OH) | borinic acid (preselected name) | |
| HB(OH)2 | boronic acid (preselected name) | |
| Br(O)2(OH) | bromic acid (preselected name) | |
| Br(O)(OH) | bromous acid (preselected name) | |
| Cl(O))2(OH) | chloric acid (preselected name) | |
| Cl(O)(OH) | chlorous acid (preselected name) | |
| Br(OH) | hypobromous acid (preselected name) | |
| Cl(OH) | hypochlorous acid (preselected name) | |
| F(OH) | hypofluorous acid (preselected name) | |
| I(OH) | hypoiodous acid (preselected name) | |
| I(O)2(OH) | iodic acid (preselected name) | |
| I(O)(OH) | iodous acid (preselected name) | |
| HO-NO2 | nitric acid (preselected name) | |
| HO-NO | nitrous acid (preselected name) | |
| Br(O)3(OH) | perbromic acid (preselected name) | |
| Cl(O)3(OH) | perchloric acid (preselected name) | |
| F(O)3(OH) | perfluoric acid (preselected name) | |
| I(O)3(OH) | periodic acid (preselected name) | |
| H2P(O)(OH) | phosphinic acid (preselected name) | |
| H2P(OH) | phosphinous acid (preselected name) | |
| HP(O)(OH)2 | phosphonic acid (preselected name) | |
| HP(OH)2 | phosphonous acid (preselected name) | |
| P(O)(OH)3 | phosphoric acid (preselected name) | |
| P(OH)3 | phosphorous acid (preselected name) | |
| Se(O)2(OH)2 | selenic acid (preselected name) | |
| Se(O)(OH)2 | selenous acid (preselected name) | |
| Si(OH)4 | silicic acid (preselected name) (not orthosilicic acid) | |
| H2Sb(O)(OH) | stibinic acid (preselected name) | |
| H2Sb(OH) | stibinous acid (preselected name) | |
| HSb(O)(OH)2 | stibonic acid (preselected name) | |
| HSb(OH)2 | stibonous acid (preselected name) | |
| Sb(O)(OH)3 | stiboric acid (preselected name) antimonic acid | |
| Note: Stiboric acid is preferred to antimonic acid as the preselected name for clarity and consistency with stibonic and with phosphoric and arsoric acid | ||
| Sb(OH)3 | stiborous acid (preselected name) antimonous acid | |
| Note: Stiborous acid is preferred to antimonous acid as the preselected name for clarity and consistency with stibonic acid and with phosphorous and arsorous acid | ||
| S(O)2(OH)2 | sulfuric acid (preselected name) | |
| S(O)(OH)2 | sulfurous acid (preselected name) | |
| Te(O)2(OH)2 | telluric acid (preselected name) | |
| Te(O)(OH)2 | tellurous acid (preselected name) | |
Acids with hydrogen atoms attached to the central atom may be substituted by organyl groups and preferred IUPAC names are formed in this manner.
Note: Another method has been suggested which would treat the acid as a suffix (like sulfonic acid) leading to names such as benzenephosphonic acid. This suggestion has been rejected because in cases where the acid has two substitutable hydrogen atoms, the use of additional letter locants would be required leading to unnecessarily more cumbersome names.
Examples:
(C2H5)2P(O)(OH)
diethylphosphinic acid (PIN)
(not P-ethylethanephosphinic acid)
(C6H5)2As(OH)
diphenylarsinous acid (PIN)
C6H5Sb(OH)2
phenylstibonous acid (PIN)
(naphthalene-2,6-diyl)bis(phosphonous acid) (PIN)
Mononuclear noncarbon oxoacids are modified by either infixes or prefixes in functional replacement nomenclature.
P-67.1.2.1 Mononuclear acids modified by infixesP-67.1.2.1 Mononuclear noncarbon oxoacids modified by infixes. The following acids are modified by infixes; they are listed in group order B, N, P, As, Sb, S, Se, Te:
P-67.1.2.2 Mononuclear acids modified by prefixes
P-67.1.2.3 General methodology for functional replacement nomenclature using infixes
P-67.1.2.4 Mononuclear noncarbon oxoacids modified by functional replacement nomenclature
P-67.1.2.5 Acid halides and pseudohalides
P-67.1.2.6 Amides and hydrazides
| B(OH)3 | boric acid | |
| HB(OH)2 | boronic acid | |
| H2B(OH) | borinic acid | |
| N(O)(OH)3 | nitroric acid (hypothetical) | |
| N(OH)3 | azorous acid (hypothetical) | |
| HN(O)(OH)2 | azonic acid | |
| H2N(O)(OH) | azinic acid | |
| HN(OH)2 | azonous acid | |
| P(O)(OH)3 | phosphoric acid | |
| P(OH)3 | phosphorous acid | |
| HP(O)(OH)2 | phosphonic acid | |
| HP(OH)2 | phosphonous acid | |
| H2P(O)(OH) | phosphinic acid | |
| H2P(OH) | phosphinous acid | |
| As(O)(OH)3 | arsoric acid (formerly arsenic acid) | |
| As(OH)3 | arsorous acid (formerly arsen(i)ous acid) | |
| HAs(O)(OH)2 | arsonic acid | |
| HAs(OH)2 | arsonous acid | |
| H2As(O)(OH) | arsinic acid | |
| H2As(OH) | arsinous acid | |
| Sb(O)(OH)3 | stiboric acid (formerly antimonic acid) | |
| Sb(OH)3 | stiborous acid (formerly antimonous acid) | |
| HSb(O)(OH)2 | stibonic acid | |
| HSb(OH)2 | stibonous acid | |
| H2Sb(O)(OH) | stibinic acid | |
| H2Sb(OH) | stibinous acid | |
| S(O)2(OH)2 | sulfuric acid | |
| S(O)(OH)2 | sulfurous acid | |
| Se(O)2(OH)2 | selenic acid | |
| Se(O)(OH)2 | selenous acid | |
| Te(O)2(OH)2 | telluric acid | |
| Te(O)(OH)2 | tellurous acid |
P-67.1.2.2 Mononuclear noncarbon oxoacids modified by prefixes. The following acids are modified by prefixes; they are listed in the order Si, N, F, Cl, Br, I.
| Si(OH)4 | silicic acid (formerly orthosilicic acid) | |
| HO-NO2 | nitric acid | |
| HO-NO | nitrous acid | |
| F(O)3(OH) | perfluoric acid | |
| F(O)2(OH) | fluoric acid | |
| F(O)(OH) | fluorous acid | |
| F(OH) | hypofluorous acid | |
| Cl(O)3(OH) | perchloric acid | |
| Cl(O)2(OH) | chloric acid | |
| Cl(O)(OH) | chlorous acid | |
| Cl(OH) | hypochlorous acid | |
| Br(O)3(OH) | perbromic acid | |
| Br(O)2(OH) | bromic acid | |
| Br(O)(OH) | bromous acid | |
| Br(OH) | hypobromous acid | |
| I(O)3(OH) | periodic acid | |
| I(O)2(OH) | iodic acid | |
| I(O)(OH) | iodous acid | |
| I(OH) | hypoiodous acid |
P-67.1.2.3 General methodology for functional replacement nomenclature using infixes
Functional replacement nomenclature (see P-15.5) using infixes generates functional class names for the following classes: acid halides and pseudo halides (azides, cyanides, isocyanides, and isocyanates), amides, hydrazides, and also imidic, hydrazonic and nitridic acids. Chalcogen analogues are also described by infixes.
Note: Preferred IUPAC names are retained names modified by functional nomenclature. The use of infixes is restricted to acids listed in P-67.1.2.1 and leads to preferred IUPAC names. Prefixes are used as recommended for acids listed in P-67.1.2.2, and in general nomenclature for all mononuclear acids. Substitutive names and names modified by prefixes are used only in special occasions (see P-67.1.4.1.1.6 and P-67.3.1).
Example:
| (1) | –OO– | peroxo |
| (2) | –OS– or –SO– | thioperoxo (similarly selenoperoxo, telluroperoxo) |
| (3) | –SS– | dithioperoxo (similarly diselenoperoxo, ditelluroperoxo) |
| (4) | –SSe– or –SeS– | selenothioperoxo (similarly for other mixed chalcogens) |
| (5) | –S– or =S | thio |
| (6) | –Se– or =Se | seleno |
| (7) | –Te– or =Te | telluro |
P-67.1.2.3.2 Infixes denoting replacement of -OH group in decreasing order of seniority with all halides (class 1) and pseudohalides (class 2) having the same rank within the class.
| (1) | –Br | bromido |
| –Cl | chlorido | |
| –F | fluorido | |
| –I | iodido | |
| (2) | –N3 | azido |
| –OCN | cyanatido | |
| –CN | cyanido | |
| –NCO | isocyanatido | |
| –NC | isocyanido | |
| –NCSe | isoselenocyanatido | |
| –NCTe | isotellurocyanatido | |
| –NCS | isothiocyanatido | |
| –SeCN | selenocyanatido | |
| –TeCN | tellurocyanatido | |
| –SCN | thiocyanatidoo | |
| (3) | –NH2 | amido |
| (4) | –NH-NH2 | hydrazido |
| (5) | ≡N | nitrido |
| (6) | =NH | imido |
| (7) | =NNH2 | hydrazono |
| (1) | –OO– | peroxo |
| (2) | –OS– or –SO– | thioperoxy (similarly selenoperoxy, telluroperoxy) |
| (3) | –SS– | dithioperoxy (similarly diselenoperoxy, ditelluroperoxy) |
| (4) | –SSe– or –SeS– | selenothioperoxy (similarly for other mixed chalcogens) |
| (5) | –S– or =S | thio |
| (6) | –Se– or =Se | seleno |
| (7) | –Te– or =Te | telluro |
P-67.1.2.3.4 Prefixes denoting replacement of -OH group in decreasing order of seniority with all halides (class 1) and pseudohalides (class 2)
having the same rank within the class.
| (1) | –Br | bromo |
| –Cl | chloro | |
| –F | fluoro | |
| –I | iodo | |
| (2) | –N3 | azido |
| –OCN | cyanato | |
| –CN | cyano | |
| –NCO | isocyanato | |
| –NC | isocyano | |
| –NCSe | isoselenocyanato | |
| –NCTe | isotellurocyanato | |
| –NCS | isothiocyanato | |
| –SeCN | selenocyanato | |
| –TeCN | tellurocyanato | |
| –SCN | thiocyanato | |
| (4) | –NH-NH2 | hydrazido |
| (5) | ≡N | nitrido |
| (6) | =NH | imido |
| (7) | =NNH2 | hydrazono |
The appropriate prefix is indicated (in alphabetical order if there is more than one) before the name of the acid; no elision is recommended. Multiplying a prefix by multiplying prefixes ‘di’ or ‘tri’ does not change its place in the alphabetical order.
P-67.1.2.4 Mononuclear noncarbon oxoacids modified by functional replacement nomenclature
Preselected names described in P-67.1.2 are used for deriving preferred IUPAC names for organic compounds. As long as there is at least one –OH group left in an oxoacid having a retained name, the acid modified by functional replacement is classified as an acid and denoted by class name ‘acid’.
P-67.1.2.4.1 Functional replacement of the oxoacids specifically listed in P-67.1.2 is expressed by infixes or prefixes. Substitution of nonacidic hydrogen atoms is indicated by prefixes, with a letter locant B, N, P, As or Sb, as needed. Tautomers may be distinguished by prefixing italic elements symbols, such as S and O, to the term ‘acid’. Parentheses are needed to enclose infixes modified by a chalcogen prefix, for example, ‘thioperoxoic’. In addition to infixes and prefixes listed in P-67.1.2.3, the prefix ‘cyanato’ and the infix ‘cyanatido’, for –OCN, are used to modify acids as indicated in P-67.1.2.4.1.3.
P-67.1.2.4.1.1 Examples of mononuclear noncarbon oxoacids modified by infixes:
CH3-B(NH-CH3)(OH)
B,N-dimethylboronamidic acid (PIN)
CH3-N(OH)(SH)
methylazonothious acid (PIN)
(C2H5)2P(S)(SH)
diethylphosphinodithioic acid (PIN)
(CH3)2N-P(O)(OH)2
dimethylphosphoramidic acid (PIN)
(C6H5)2P(=N-CH3)(OH)
N-methyl-P,P-diphenylphosphinimidic acid (PIN)
C6H5-P(=N-C6H5)(Cl)(SH)
N,P-diphenylphosphonochloridimidothioic acid (PIN)
C6H5-P(S)(NH-CH3)(OH)
N-methyl-P-phenylphosphonamidothioic O-acid (PIN)
(CH3)2N-P(O)(NCS)(SH)
N,N-dimethylphosphoramid(isothiocyanatido)thioic S-acid (PIN)
(CH3)2N-P(=N-C6H5)(SCN)(OH)
N,N-dimethyl-N′-phenylphosphoramidimido(thiocyanatidic) acid (PIN)
C6H5-P(OH)(SH)
phenylphosphonothious acid (PIN)
C6H5-P(≡N)(OH)
phenylphosphononitridic acid (PIN)
C6H5-P(O)(Cl)(OH)
phenylphosphonochloridic acid (PIN)
CH3-CH2-P(Se)(OH)2
ethylphosphonoselenoic O,O-acid (PIN)
CH3-CH2-P(O)(OH)(SeH)
ethylphosphonoselenoic Se-acid (PIN)
P(=NH)(NH-NH2)(OH)2
phosphorohydrazidimidic acid
(name derived from the preselected name phosphoric acid)
P(O)(OH)(SH)(SSH)
phosphoro(dithioperoxo)thioic S-acid
(name derived from the preselected name phosphoric acid)
P(O)(OH)2(OSH)
phosphoro(thioperoxoic) OS-acid
(name derived from the preselected name phosophoric acid)
As(O)(OH)(SH)2 or As(S)(OH)2(SH)
arsorodithioic acid
(name derived from the preselected name arsoric acid)
As(S)(OH)3
arsorothioic O,O,O-acid
(name derived from the preselected name arsoric acid)
(C6H5)2As(SH)
diphenylarsinothious acid (PIN)
HO-SO2-SH
sulfurothioic S-acid
(name derived from the preselected name sulfuric acid)
H2N-SO2-OH
sulfamic acid
(name derived from the preselected name sulfuric acid; contraction of sulfuramidic acid)
H2S2O3
sulfurothioic acid
(name derived from the preselected name sulfuric acid; the position of the sulfur atom is undetermined)
HO-SO2-NC
sulfurisocyanidic acid (PIN)
HO-SO2-NCS
sulfur(isothiocyanatidic) acid (PIN)
HO-SO2-CN
sulfurocyanidic acid (PIN)
HS-SO2-NH2
sulfamothioic S-acid
(name derived from the preselected name sulfuric acid; a contraction of sulfuramidothioic S-acid)
HS-TeO2-NH2
telluramidothioic acid
(name derived from the preselected name telluric acid)
S=N-OH
thionitrous O-acid
(name derived from the preselected name nitrous acid)
Cl(S)2-OH
dithiochloric O-acid
(name derived from the preselected name chloric acid)
When attached to the central atom of a mononuclear noncarbon oxoacid, the group –OCN creates an anhydride linkage (see P-67.1.3.3). In order to respect the seniority order classes, this group is used, therefore, in functional replacement nomenclature to name acids; acids are senior to anhydrides. For the prefixes ‘cyanato’, ‘thiocyanato’, ‘selenocyanato’ and ‘tellurocyanato’, see P-65.2.2.
Examples:
P(O)(OCN)2OH
phosphorodicyanatidic acid (PIN)
Si(OCN)(OH)3
cyanatosilicic acid (PIN)
The names phosphonous, phosphinous, phosphonic and phosphinic acid (and similarly for arsenic, antimony and nitrogen acids) can only be used when P, As or Sb is attached to atoms of hydrogen, carbon or another atom of a parent hydride such as N, As, Si. Thus, C6H5-P(O)Cl(OH) is phenylphosphonochloridic acid and not chloro(phenyl)phosphinic acid; (C5H10N)-P(O)Cl(OH) is (piperidin-1-yl)phosphonochloridic acid and not chloro(piperidin-1-yl)phosphinic acid; and ClP(O)(OH)2 is phosphorochloridic acid and not chlorophosphonic acid.
P-67.1.2.5 Acid halides and pseudohalides
P-67.1.2.5.1 Except for the boron acids and silicic acid, preferred IUPAC names of acid halides and pseudohalides are formed by adding the class name(s) of a halide or pseudohalide to that of the acid. Exceptionally, in accordance with tradition and the recommended nomenclature of inorganic compounds (ref. 12), halides and pseudohalides with identical atoms or groups and derived from phosphoric acid, sulfuric acid, selenic acid and telluric acid are named by adding the class name(s) to the acyl group name ‘phosphoryl’, ‘sulfuryl’, ‘sulfamoyl’, ‘selenonyl’ and ‘telluronyl’, and not to the name of the acid itself. In accordance with the seniority order of halides and pseudohalides, names are formed on the basis of the senior class, as described in P-67.1.2.1.
Preferred IUPAC names of acid halides and pseudohalides derived from the boron acids and silicic acid are formed on the basis of the parent hydride names borane and silane, respectively.
Examples:
P(O)(NCO)3
phosphoryl triisocyanate (PIN)
(C6H5)2P-Cl
diphenylphosphinous chloride (PIN)
(C6H5)2Sb-NCO
diphenylstibinous isocyanate (PIN)
C6H5-PCl2
phenylphosphonous dichloride (PIN)
C6H5-PBrCl
phenylphosphonous bromide chloride (PIN)
phenylphosphonobromidous chloride
(C6H5)2P(=N-C6H5)Cl
N,P,P-triphenylphosphinimidic chloride (PIN)
(CH3-CH2)2P(S)Cl
diethylphosphinothioic chloride (PIN)
C6H5-P(O)Cl2
phenylphosphonic dichloride (PIN)
CH3-CH2-P(O)[N(CH3)2]Cl
P-ethyl-N,N-dimethylphosphonamidic chloride (PIN)
(CH3)2N-P(O)(NCO)Cl
N,N-dimethylphosphoramidisocyanatidic chloride (PIN)
HP(O)(NCO)2
phosphonic diisocyanate (PIN)
P(=NH)(NCS)3
phosphorimidic triisothiocyanate (PIN)
(CH3)2PN3
dimethylphosphinous azide (PIN)
SO2(NCO)2
sulfuryl diisocyanate (PIN)
S(=N-CH3)Cl2
N-methylsulfurimidous dichloride (PIN)
F-SO2-NCO
sulfurisocyanatidic fluoride (PIN)
F-S(=NH)(NCO)
sulfurimidisocyanatidous fluoride (PIN)
CH3-NH-SO2Cl
N-methylsulfamoyl chloride (PIN)
Examples:
CH3-SiCl3
trichloro(methyl)silane (PIN)
SiCl4
tetrachlorosilane (preselected name)
silicon tetrachloride
(not silicic tetrachloride)
Amides and hydrazides are named by functional class nomenclature by replacing the term ‘acid’ in the name of the corresponding acid by ‘amide’ or ‘hydrazide’. Amides and hydrazides of nitric acid and nitrous acid are discussed in P-67.1.2.6.3. Preferred IUPAC names of amides and hydrazides of the boron acids and silicic acid are exceptions (see P-67.1.2.6.2) as are azorous acid, azinous acid and azonous acid which are names of polyazanes.
P-67.1.2.6.1 Preferred IUPAC names of amides and hydrazides are denoted by the class name ‘amide’ or ‘hydrazide’:
(a) when all –OH groups in the corresponding acid have been replaced by –NH2 or –NH-NH2 groups, andBr, Cl, F, I, N3, CN, NC, NCO, ONC, NH2 (amide),(b) when the amide or hydrazide is the principal functional group in accordance with the following order of seniority:
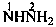 (hydrazide)
(hydrazide)Note: This order is not quite the same as that used by CAS (where amide follows the halogens and precedes the pseudohalogens) but it is consistent with the order of compound classes in P-41.
Substituents on the nitrogen atoms are denoted by italic letter locants such as, N (primed and double primed as required), in addition to the italic letter locants ‘P’, ‘As’, and ‘Sb’.
Locants to denote hydrazides are 1 and 2, primed and double primed as required.
Examples:
(CH3)2P(O)[N(CH3)2]
N,N,P,P-tetramethylphosphinic amide (PIN)
(not dimethylphosphinic dimethylamide)
C6H5-P(O)(NHCH3)2
N,N′-dimethyl-P-phenylphosphonic diamide (PIN)
[not phenylphosphonic bis(methylamide)]
C6H5-P(S)[N(CH3)2]2
N,N,N′,N′-tetramethyl-P-phenylphosphonothioic diamide (PIN)
C6H5-Sb(S)[N(CH3)2][N(CH2-CH3)2]
N,N-diethyl-N′,N′-dimethyl-Sb-phenylstibonothioic diamide (PIN)
(CH3)2N-P(O)Cl2
N,N-dimethylphosphoramidic dichloride (PIN)
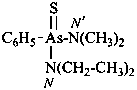
N,N-diethyl-N′,N′-dimethyl-As-phenylarsonothioic diamide (PIN)
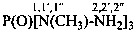
1,1′,1′′-trimethylphosphoric trihydrazide (PIN)
2,2,2′,2′,2′′,2′′-hexamethylphosphorothioic trihydrazide (PIN)
[not phosphorothioic tris(2,2-dimethylhydrazide)]
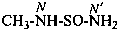
N-methylsulfurous diamide (PIN)
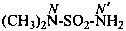
N,N-dimethylsulfuric diamide (PIN)
N,N-dimethylsulfamide
pentamethylsulfurimidous diamide (PIN)
CH3-NH-S(O)(=N-CH3)-Br
N,N′-dimethylsulfuramidimidic bromide (PIN)
N,N,N′,N′-tetramethyl-N′′-phenylsulfurimidic diamide (PIN)
Examples:
B(NH2)3
boranetriamine
(name derived from the preselected name borane)
(not boric triamide)
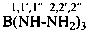
1,1′,1′′-boranetriyltrihydrazine
(name derived from the preselected name borane)
(not boric trihydrazide)
Si(NH2)4
silanetetramine
(name derived from the preselected name silane)
(not silicic tetramide)
Si(NH-NH2)4
1,1′,1′′,1′′′-silanetetrayltetrahydrazine
(name derived from the preselected name silane )
(not silicic tetrahydrazide)
Nitramines are amides of nitric acid (see ref. 23). The class is composed of ‘nitramide’ (a shortened form of nitric amide), NO2-NH2, and the names of its derivatives are formed by substitution. Nitrosamines are amides of nitrous acid, NO-NH2 (see ref. 23); and the names of its derivatives are formed by substitution. Nitric acid and nitrous acid are preselected names, see P-12.
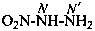
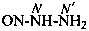
(II)
Preferred IUPAC names for amides and hydrazides of nitric and nitrous acids are now systematically based on nitric or nitrous amide and hydrazide, in accordance with the seniority order of classes rather than as nitro and nitroso amines; the latter names can be used in general nomenclature.
Examples:
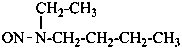
butyl(ethyl)nitrous amide (PIN)
N-ethyl-N-nitrosobutan-1-amine
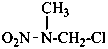
(chloromethyl)(methyl)nitramide (PIN)
1-chloro-N-methyl-N-nitromethanamine
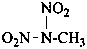
methyl(nitro)nitramide (PIN)
N,N-dinitromethanamine
N′-hexylidenenitrous hydrazide (PIN)
The methodology discussed in this section is applicable to all mononuclear oxo acids whether or not they have retained names or names using infixes or prefixes.
P-67.1.3.1 SaltsP-67.1.3.1 Salts of mononuclear noncarbon oxoacids
P-67.1.3.2 Esters
P-67.1.3.3 Anhydrides
Neutral salts of mononuclear noncarbon oxoacids are named by citing the cation(s) followed by the name(s) of the anion(s) as a separate word. Names of anions are formed by changing the ‘ic acid’ ending to ‘ate’ and the ‘ous acid’ ending to ‘ite’. Different cations are cited in alphabetical order.
Examples:
K[(CH3)2As(O)O]
potassium dimethylarsinate (PIN)
Note: In the nomenclature of inorganic chemistry (IR-8.4, ref. 12), the term ‘hydrogen’ is written directly in front of the name of the anion, without a space, to indicate that it is part of the anion.
Example:
Esters of mononuclear noncarbon acids are named in the same way as esters of organic acids (see P-65.6.3.2). Alkyl groups, aryl groups, etc. are cited as separate words, in alphanumerical order when more than one, and followed by the name of the appropriate anion. Partial acid esters of polybasic acids are named by citing alkyl groups, aryl groups, etc. as separate words, in alphanumeric order if more than one, followed by the word ‘hydrogen’ (with the appropriate multiplying prefix, as necessary) also cited as a separate word, and the name of the appropriate anion. Salts of partial acid esters are named by citing the name of the cation before the name of the organic group; remaining acids groups are denoted by the word ‘hydrogen’ as described above. Structural specificity for esters of chalcogen analogues of mononuclear noncarbon oxoacids is provided by the appropriate italic element symbols O, S, Se, and Te, prefixed to the name of the group, as needed.
Examples:
CH3-S-NO2
S-methyl thionitrate (PIN)
(C6H5)2P-O-CH3
methyl diphenylphosphinite (PIN)
CH3-P(Cl)(S-CH2-CH3)
ethyl methylphosphonochloridothioite (PIN)
CH3-P(NH-CH3)(OCH3)
methyl N,P-dimethylphosphonamidite (PIN)
P(O-CH3)3
trimethyl phosphite (PIN)
P(Cl)[N(CH3)2](O-CH3)
methyl N,N-dimethylphosphoramidochloridite (PIN)
P(O)(O-CH3)3
trimethyl phosphate (PIN)
P(O)(O-C2H5)(O-CH3)(O-C6H5)
ethyl methyl phenyl phosphate (PIN)
P(O)(O-CH3)(OH)2
methyl dihydrogen phosphate (PIN)
C6H5-HAs(S)(O-CH3)
O-methyl phenylarsinothioate (PIN)
CH3-O-P(O)(OH)-O– Na+
sodium methyl hydrogen phosphate (PIN)
HP(O)(O-CH3)2
dimethyl phosphonate (PIN)
(CH3-CH2)2P(S)(S-CH2-CH3)
ethyl diethylphosphinodithioate (PIN)
(CH3)2As(O)(S-CH3)
S-methyl dimethylarsinothioate (PIN)
CH3-P(O)(O-CH2-CH3)2
diethyl methylphosphonate (PIN)
C6H5-P(O)(O-CH3)(S-CH2-CH3)
S-ethyl O-methyl phenylphosphonothioate (PIN)
C6H5-P(O)(Cl)(O-CH3)
methyl phenylphosphonochloridate (PIN)
(CH3)2N-P(O)(O-CH3)2
dimethyl N,N-dimethylphosphoramidate (PIN)
(CH3-CH2)2N-P(O)(NCS)(O-CH2-CH3)
ethyl N,N-diethylphosphoramid(isothiocyanatidate) (PIN)
As(O)(F)(O-CH3)2
dimethyl arsorofluoridate (PIN)
Sb(O)(F)2(S-CH3)
S-methyl stiborodifluoridothioate (PIN)
(CH3)2B-O-C6H5
phenyl dimethylborinate (PIN)
CH3-O-SO2-OH
methyl hydrogen sulfate (PIN)
C6H5-O-F
phenyl hypofluorite (PIN)
CH3-S-Cl
methyl thiohypochlorite (PIN)
CH3-CO-CH2-CH2-O-BrO2
3-oxobutyl bromate (PIN)
methyl bis(1-hydroxybutan-2-yl)borinate (PIN)
Si(S-CH3)3(O-CH2-CH3)
O-ethyl S,S,S-trimethyl trithiosilicate (PIN)
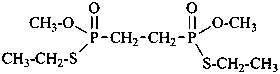
S,S′-diethyl O,O′-dimethyl P,P′-(ethane-1,2-diyl)bis(phosphonothioate)(PIN)
Neutral anhydrides formed between acids named by suffixes and mononuclear noncarbon oxoacids described in P-67.1.1 are named in the same way as described for anhydrides derived from carboxylic and the sulfur acids named by suffixes (see P-65.7). The names of the acids are cited in alphabetical order followed by the class name ‘anhydride’ prefixed by a numerical term indicating the number of anhydride linkages (the numerical prefix mono is not used); such names lead to preferred IUPAC names. Names formed by acyl groups substituting the phosphorus, arsenic or antimony mononuclear oxoacid modified by the ending ‘ate’ can be used in general nomenclature. Halogen oxoacids form anhydrides with carboxylic and the sulfur acids expressed by suffixes.
Acidic anhydrides are named as described in P-67.3.1 using the senior acid as parent, or by using systematic substitutive nomenclature.
Examples:
CH3-CO-O-As(O)(CH3)2
acetic dimethylarsinic anhydride (PIN)
acetyl dimethylarsinate
B(O-CO-CH3)3
acetic boric trianhydride (PIN)
[(CH3)2CH]2Sb-S-C(S)-N(CH2-CH3)2
diethylcarbamothioic di(propan-2-yl)stibinous thioanhydride (PIN)
C6H5-CO-O-I
benzoic hypoiodous anhydride (PIN)
(CH3)2B-O-O-B(CH3)2
dimethylborinic peroxyanhydride (PIN)
B(OCN)3
boric cyanic trianhydride (PIN)
CH3-HP(O)(OCN)
cyanic methylphosphinic anhydride (PIN)
P-67.1.4.1 Substituent groups derived from the mononuclear nitrogen, phosphorus, arsenic, and antimony acidsP-67.1.4.1 Substituent groups derived from the mononuclear nitrogen, phosphorus, arsenic, and antimony acids
P-67.1.4.2 Substituent groups derived from boron acid and silicic acid
P-67-1.4.3 Substituent groups derived from nitric and nitrous acids
P-67.1.4.4 Substituent groups derived from chalcogen acids
P-67.1.4.5 Substituent groups derived from halogen acids
P-67.1.4.1.1 Preselected prefixesP-67.1.4.1.1 Preselected prefixes.
P-67.1.4.1.2 Substituent groups for general nomenclature
P-67.1.4.1.3 Compound and complex substituent groups
Substituent prefix groups derived from mononuclear noncarbon oxoacids have retained names and systematic names corresponding to simple or compound acyl groups. The preselected prefixes are formed by applying the following seniority order, in the order given, until a decision is reached:
P-67.1.4.1.1.1 Retained names for substituent groups derived from mononuclear noncarbon oxoacids;
A few names denoting monovalent acidic groups are retained. These names are preselected names when unsubstituted or for chalcogen analogues when the position of chalcogen atoms introduced by functional replacement is not known or it is not necessary to specify their position(s): the chalcogen atoms are expressed by prefixes.
| Acid | Derived preselected prefix |
| –N(O)(OH)2 | azono (preselected prefix) |
| –P(O)(OH)2 | phosphono (preselected prefix) |
| –As(O)(OH)2 | arsono (preselected prefix) |
| –Sb(O)(OH)2 | stibono (preselected prefix) |
| –P(O)(OH)(SH) or –P(S)(OH)2 | thiophosphono (preselected prefix) |
| –P(S)(SH)2 | trithiophosphono (preselected prefix) |
P-67.1.4.1.1.2 Fundamental acyl groups for substituents derived from mononuclear noncarbon oxoacids;
Acyl prefix groups are formed by removing all –OH groups from a mononuclear noncarbon oxoacid having the general structures E(=O)(OH)3, R-E(=O)(OH)2 or R,R′E(=O)OH, where R and R′ = H or an organyl group. Names of acyl groups derived from the names of acids, modified or not by functional replacement, by elimination of all hydroxy groups or their chalcogen analogues, are formed by changing the ‘ic acid’ ending in the name of the acid to ‘oyl’, with the exception of ‘nitroryl’ –N(O)<; ‘phosphoryl’, –P(O)<; ‘arsoryl’, –As(O)<; and ‘stiboryl’, –Sb(O)<. Prefixes formed in this manner are preselected prefixes. For example, the group ‘phosphoryl’ –P(O)<, is derived from phosphoric acid, P(O)(OH)3 or phosphorothioic S-acid, P(O)(OH)2(SH), or phosphorodithioic S,S-acid, P(O)(SH)2(OH) or phosphorotrithioic S,S,S-acid, P(O)(SH)3.
The prefix ‘nitroryl’ (not azoryl), for the acyl group –N(O)<, derived from the hypothetical nitroric acid, N(O)(OH)3, has been recommended since 1993 (see R-3.3, ref. 2).
Examples:
| Acid | Derived preselected prefix | |||
| N(O)(OH)3 | nitroric acid (hypothetical; preselected name) | –N(O)< | nitroryl (preselected prefix) | |
| P(O)(OH)3 | phosphoric acid (preselected name) | –P(O)< | phosphoryl (preselected prefix) | |
| As(O)(OH)3 | arsoric acid (preselected name) (not arsenic acid) | –As(O)< | arsoryl (preselected prefix) (not arsenyl) | |
| Sb(O)(OH)3 | stiboric acid (preselected name) (not antimonic acid) | –Sb(O)< | stiboryl (preselected prefix) (not antimonyl) | |
| NH(O)(OH)2 | azonic acid (preselected name) | NH(O)< | azonoyl (preselected prefix) | |
| NH2(O)(OH) | azinic acid (preselected name) | NH2(O)– | azinoyl (preselected prefix) | |
| PH(O)(OH)2 | phosphonic acid (preselected name) | PH(O)< | phosphonoyl (preselected prefix) | |
| PH2(O)(OH) | phosphinic acid (preselected name) | PH2(O)– | phosphinoyl (preselected prefix) (not phosphinyl) | |
| AsH(O)(OH)2 | arsonic acid (preselected name) | AsH(O)< | arsonoyl (preselected prefix) | |
| AsH2(O)OH | arsinic acid (preselected name) | AsH2(O)– | arsinoyl (preselected prefix (not arsinyl) | |
| SbH(O)(OH)2 | stibonic acid (preselected name) | SbH(O)< | stibonoyl (preselected prefix) | |
| SbH2(O)OH | stibinic acid (preselected name) | SbH2(O)– | stibinoyl (preselected prefix) | |
P-67.1.4.1.1.3 Names of substituted fundamental acyl groups derived from mononuclear noncarbon oxoacids
Names of substituted fundamental acyl groups are formed directly from those of the acids generated by the method described in P-67.1.4.1.1.2. Preferred prefix names of acyl groups are those derived from the preferred IUPAC names of acids. The addition of hydrogen atoms to acyl groups by the method of concatenation, described in P-67.1.4.1.2, is not allowed.
Examples:
| Acid | Derived preferred prefix | |
| CH3-P(O)(OH)2 methylphosphonic acid (PIN) | CH3-P(O)< methylphosphonoyl (preferred prefix) | |
| CH3-CH2-SbH(O)OH ethylstibinic acid (PIN) | CH3-CH2-SbH(O)– ethylstibinoyl (preferred prefix) | |
| C6H5-As(CH3)(O)OH methyl(phenyl)arsinic acid (PIN) | C6H5-As(CH3)(O)– methyl(phenyl)arsinoyl (preferred prefix) |
P-67.1.4.1.1.4 Names of acyl groups derived from mononuclear noncarbon oxoacids modified by functional replacement nomenclature
Preferred IUPAC prefixes are formed by the methodology indicated in P-65.2.1.5 for acyl groups derived from carbonic acids modified by the infixes and prefixes in functional replacement nomenclature. All infixes and prefixes listed in Table 1.6 and cited in P-65.1.2.3 are allowed. Applied to the B, N, P, As and Sb mononuclear noncarbon oxoacids, the method consists of first achieving functional replacement in acids, then removing all remaining –OH groups. Preselected names use infixes to denote functional replacement. Names using prefixes to effect functional replacement may be used in general nomenclature.
Examples:
| Acid | Derived preselected or preferred prefix | |
| P(S)(OH)3 phosphorothioic O,O,O-acid (preselected name) thiophosphoric O,O,O-acid | >P(S)– phosphorothioyl (preselected prefix) thiophosphoryl | |
| As(=NH)(OH)3 arsorimidic acid (preselected name) imidoarsoric acid | >As(=NH)– arsorimidoyl (preselected prefix) imidoarsoryl | |
| Sb(=NNH2)(OH)3 stiborohydrazonic acid (preselected name) hydrazonostiboric acid | >Sb(=NNH2)– stiborohydrazonoyl (preselected prefix) hydrazonostiboryl | |
| NH(S)(OH)2 azonothioic acid (preselected name) thioazonic acid | >NH(S) azonothioyl (preselected prefix) thioazonoyl | |
| PH2(=NH)(OH) phosphinimidic acid (preselected name) imidophosphinic acid | –PH2(=NH) phosphinimidoyl (preselected prefix) imidophosphinoyl | |
| (CH3)2P(Se)(OH) dimethylphosphinoselenoic acid (PIN) dimethyl(selenophosphinic acid) | (CH3)2P(Se)– dimethylphosphinoselenoyl (preferred prefix) dimethyl(selenophosphinoyl) | |
| C6H5-P(O)Cl(OH) phenylphosphonochloridic acid (PIN) phenyl(chlorophosphonic acid) | C6H5-P(O)(Cl)– phenylphosphonochloridoyl (preferred prefix) phenyl(chlorophosphonoyl) | |
| P(≡N)(OH)2 phosphoronitridic acid (preselected name) nitridophosphoric acid | >P(≡N) phosphoronitridoyl (preselected prefix) nitridophosphoryl | |
| P(=NH)(NHNH2)(OH)2 phosphorohydrazidimidic acid (preselected name) hydrazidimidophosphoric acid | >P(=NH)(NHNH2) phosphorohydrazidimidoyl (preselected prefix) hydrazidimidophosphoryl | |
| P(O)Cl2(OH) phosphorodichloridic acid (preselected name) dichlorophosphoric acid | P(O)(Cl)2– phosphorodichloridoyl (preselected prefix) dichlorophosphoryl | |
| (CH3)2N-P(O)(OH)2 N,N-dimethylphosphoramidic acid (PIN) (dimethylamido)phosphoric acid | (CH3)2N-P(O)< N,N-dimethylphosphoramidoyl (preferred prefix) (dimethylamido)phosphoryl | |
| P(O)(OH)2(OOH) phosphoroperoxoic acid (preselected prefix) peroxyphosphoric acid | >P(O)(OOH) phosphoroperoxoyl (preselected prefix) (hydroperoxy)phosphoryl peroxyphosphoryl | |
| P(O)(OH)2(OSH) or P(O)(OH)2(SOH) phosphoro(thioperoxoic) acid (preselected name) (thioperoxy)phosphoric acid | >P(O)(OSH) or >P(O)(SOH) phosphoro(thioperoxoyl) (preselected prefix) (thiohydroperoxy)phosphoryl |
P-67.1.4.1.1.5 Names for preferred or preselected prefixes of acyl groups substituted by hydroxy groups or their chalcogen and peroxy analogues.
Concatenation is the recommended method to reintroduce groups and their chalcogen analogues in substituent groups or groups that are not treated as infixes in functional replacement nomenclature, such as ‘–OR’, ‘–SR’, etc. It is important to respect the concatenation procedure that is an additive operation using acyl groups only. The following acyl groups are allowed: basic acyl groups described in P-67.1.4.1.1.2 above, substituted basic acyl groups described in P-67.1.4.1.1.3 above, and acyl groups modified by functional replacement described in P-67.1.4.1.1.4 above. Substitution of hydrogen atoms attached to the central atom (substitutable hydrogen) in the basic acyl groups described in P-67.1.4.1.1.2 above is not allowed to generate preferred IUPAC names.
This method is not recommended to generate any of the prefixes in P-67.1.4.1.1.1 above.
Examples:
–P(Se)(OCH3)2
dimethoxyphosphoroselenoyl (preferred prefix)
dimethoxy(selenophosphoryl)
–P(O)(OH)(SH)
hydroxy(sulfanyl)phosphoryl (preselected prefix)
–P(O)(SH)2
bis(sulfanyl)phosphoryl (preselected prefix)
–PH(O)(SeH)
selanylphosphonoyl (preselected prefix)
–PH(S)(SH)
sulfanylphosphonothioyl (preselected prefix)
sulfanyl(thiophosphonoyl)
>P(O)(OSH)
(sulfanyloxy)phosphoryl (preselected prefix)
(SO-thiohydroperoxy)phosphoryl
>P(S)(SOH)
(hydroxysulfanyl)phosphorothioyl (preselected prefix)
(OS-thiohydroperoxy)phosphorothioyl
CH3-P(O)(OH)–
hydroxy(methylphosphonoyl) (preferred prefix)
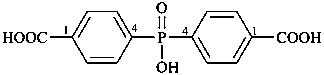
4,4′-(hydroxyphosphoryl)dibenzoic acid (PIN)
Substitutive nomenclature based on parent hydrides BH3, PH3, AsH3, SbH3, PH5, AsH5, SbH5 is used to generate names of substituent groups for which acyl group names cannot be generated by the methods in P-67.1.4.1.1.1 through P-67.1.4.1.1.5 and the corresponding As and Sb acids. It is also used to indicate a different type of free valencies, for example an ‘ylidene’ type instead of the ‘diyl’ observed in a substituent group derived from the acid in criteria (b) and (d).
Examples:
–AsH(OH)
hydroxyarsanyl (preselected prefix)
–AsHCl
chloroarsanyl (preselected prefix)
–P(NH2)2
diaminophosphanyl (preselected prefix)
>Sb(OH)
hydroxystibanediyl (preselected prefix)
=P(OH)
hydroxyphosphanylidene (preselected prefix)
=B(O-CH3)
methoxyboranylidene (preferred prefix)
–P(O-CH3)2
dimethoxyphosphanyl (preferred prefix)
=P(O)(OH)
hydroxy(oxo)-λ5-phosphanylidene (preselected prefix)
=As(O)(OCH3)
methoxy(oxo)-λ5-arsanylidene (preferred prefix)
=N(O)OH
hydroxy(oxo)-λ5-azanylidene (preselected prefix)
aci-nitro
(see P-67.1.6)
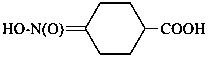
4-[hydroxy(oxo)-λ5-azanylidene]cyclohexane-1-carboxylic acid (PIN)
4-aci-nitrocyclohexane-1-carboxylic acid
(see aci-nitro compounds, P-67.1.6)
–P=O
oxophosphanyl (preselected prefix)
–P(O)2
dioxo-λ5-phosphanyl (preselected prefix)
–Sb=O
oxostibanyl (preselected prefix)
The prefix ‘hydro’ may be added by concatenation only for use in general nomenclature.
Examples:
>PH(S)
hydro(thiophosphoryl)
phosphonothioyl (preselected prefix)
If a B, N, P, As, or Sb containing group is attached by an oxygen or other chalcogen atom or a nitrogen atom to a compound that also contains another substituent having priority over the B, N, P, As, or Sb containing group for citation as principal group, then the B, N, P, As, or Sb containing group is named by a compound or complex prefix built from prefixes described above and arranged in the order in which the components occur in the compound.
Examples:
(CH3O)2P(O)-S-CH2-CH2-COOH
3-[(dimethoxyphosphoryl)sulfanyl]propanoic acid (PIN)
(HO)(HS)P(S)-NH-CH2-CH2-COOH
3-{[hydroxy(sulfanyl)phosphorothioyl]amino}propanoic acid (PIN)
Substituent groups derived from boron and silicon mononuclear acids and their analogues are formed by substituting the parent hydride names ‘borane’ and ‘silane’. The name borono, for –B(OH)2, is retained and is the preselected name.
The name boryl has been used for the substituent group H2B–, now named boranyl as the preselected name, and consequently is not to be used for the prefix group derived from boric acid by removal of all three –OH groups.
Examples:
–BH2
boranyl (preselected prefix)
(not boryl)
>BH
boranediyl (preselected prefix)
(not boronoyl)
–B<
boranetriyl (preselected prefix)
(not boryl)
=BH
boranylidene (preselected prefix)
(not boronoyl)
≡B
boranylidyne (preselected prefix)
(not boryl)
–B(NH2)2
diaminoboranyl (preselected prefix)
(not borodiamidoyl)
–BH(O-CH3)
methoxyboranyl (preferred prefix)
(not hydromethoxyboryl)
–Si(OH)3
trihydroxysilyl (preselected prefix)
–SiCl2(NH2)
aminodichlorosilyl (preselected prefix)
–Si(O-CH3)3
trimethoxysilyl (preferred prefix)
–Si(OH)2(SH)
dihydroxy(sulfanyl)silyl (preselected prefix)
The acyl groups ‘nitro’ for –NO2 and ‘nitroso’ for –NO when attached to the following elements C, P, As, Sb, Bi, Si, Ge, Sn, Pb, B, Al, Ga, In, and Tl were discussed in P-61.5. In this section, the rules for naming substituent groups related to esters, amides and hydrazides of nitric acid and nitrous acid are discussed.
P-67.1.4.3.1 Substituent groups derived from esters of nitric acid and nitrous acid
Nitrates and nitrites as esters of nitric acid and nitrous acid are discussed in P-67.1.3.2. Substituent groups derived from these compounds are named by concatenation by adding the acyl groups ‘nitro’ for –NO2 and ‘nitroso’ for –NO to the prefix ‘oxy’ or by substituting these acyl groups into substituent groups such as ‘sulfanyl’ , ‘selanyl’, or ‘tellanyl’.
Examples:
–S-NO2
nitrosulfanyl (preselected prefix)
–O-NO
nitrosooxy (preselected prefix
–Se-NO
nitrososelanyl (preselected prefix)
The amide of nitric acid, O2N-NH2, is named ‘nitramide’ and the substituent group derived from this amide by the loss of one hydrogen atom is called ‘nitramido’ by applying the general rule for naming amides (see P-66.1.1.4.3); that is, changing the final letter ‘e’ of the amide name to ‘o’. Other derivatives and those derived from nitrous amide, ON–NH2, are formed by substituting the acyl groups ‘nitro’ and ‘nitroso’ into the appropriate substituent groups ‘amino’, ‘imino’, and ‘azanetriyl’. It must be noted that the location of the groups –NO and –NO2 to another nitrogen atom does not constitute lengthening of a chain.
Examples:
>N-NO2
nitroazanediyl (preselected prefix)
=N-NO2
nitroimino (preselected prefix)
–NH-NO2
nitramido (preselected prefix)
–N=S
sulfanylideneamino (preselected prefix)
thioxoamino
thionitroso
–O2S-N=S
(sulfanylideneamino)sulfonyl (preselected prefix)
(thioxoimino)sulfonyl
(thionitroso)sulfonyl
Substituent groups derived from nitric hydrazide, O2N-NH-NH2, and nitrous hydrazide, ON-NH-NH2, are named by substituting the acyl groups ‘nitro’ or ‘nitroso’ into the appropriate substituent group ‘hydrazin-1-yl’, ‘hydrazin-1-ylidene’, etc.
Examples:
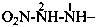
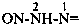
nitrosohydrazinylidene (preselected prefix)
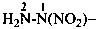
1-nitrohydrazin-1-yl (preselected prefix)
P-67.1.4.4.1 Acyl groups
There are two methods to generate names of acyl groups derived from the chalcogen acids for use as substituent groups.
(1) The methodology described in P-65.3.2.3, i.e., infixes denoting functional replacement by -S-, -Se-, -Te-, =NH, and =NNH2, and concatenation for other atoms or groups.Method (1) produces preselected prefixes.(2) The general method described in P-65.2.1.5 for deriving acyl groups from derivatives of carbonic acid, and applied in P-67.1.4.1.1.4 for acyl groups derived from phosphoric, phosphonic and phosphinic acids and their arsenic and antimony congeners is applied to sulfuric acid and its functional replacement analogues.
Examples:
–S(O)(S)–
sulfonothioyl (preselected prefix)
sulfurothioyl
–S(S)2–
sulfonodithioyl (preselected prefix)
sulfurodithioyl
–S(O)(=NH)–
sulfonimidoyl (preselected prefix)
sulfurimidoyl
–S(=NH)2–
sulfonodiimidoyl (preselected prefix)
sulfurodiimidoyl
–S(O)(=NNH2)–
sulfonohydrazonoyl (preselected prefix)
sulfurohydrazonoyl
–S(=NNH2)2–
sulfonodihydrazonoyl (preselected prefix)
sulfurodihydrazonoyl
–SO2-Cl
chlorosulfonyl (preselected prefix)
sulfurochloridoyl
–SO2-CN
cyanosulfonyl (preferred prefix)
sulfurocyanidoyl
–SO2-NCS
isothiocyanatosulfonyl (preferred prefix)
sulfur(isothiocyanatidoyl)
–S(O)(S)-NCS
isothiocyanatosulfonothioyl (preferred prefix)
sulfur(isothiocyanatido)thioyl
–SO2-O-CH3
methoxysulfonyl (preferred prefix)
methoxysulfuryl
–S(=O)-Cl
chlorosulfinyl (preselected prefix)
P-67.1.4.4.2 If a sulfur-containing group is attached by oxygen (chalcogen) or nitrogen to a compound that contains also another substituent having priority over the sulfur- containing group for citation as principal group, then the sulfur-containing group is named by an appropriate prefix formed by concatenation or substitution (see P-35.4) as described in P-65.3.2.3 and P-67.1.4.4.1.
Examples:
3-[(methoxysulfinyl)oxy]propanoic acid (PIN)
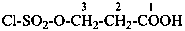
3-[(chlorosulfonyl)oxy]propanoic acid (PIN)
3-(sulfurochloridoyloxy)propanoic acid
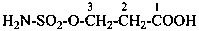
3-(sulfamoyloxy)propanoic acid (PIN)
3-(sulfuramidoyloxy)propanoic acid
[not 3-(sulfonamidoyloxy)propanoic acid;
the name sulfonamidic acid is not an approved name]
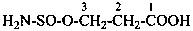
3-[(aminosulfinyl)oxy]propanoic acid (PIN)
[not 3-(sulfinamidoyloxy)propanoic acid;
the name sulfinamidic acid is not an approved name]
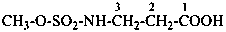
3-[(methoxysulfonyl)amino]propanoic acid (PIN)
Names of prefixes derived from halogen acids and their chalcogen analogues are used as compulsory prefixes in substitutive nomenclature. They are listed in Table 5.1 and discussed in P-61.3.2.3.
Examples:
SCl–
thiochlorosyl (preselected prefix)
O2Cl– chloryl (preselected prefix)
O3Cl–
perchloryl (preselected prefix)
Example:
P-67.1.5.1 When a characteristic group having priority to be cited as principal group is present (see seniority order of classes, P-41, and of acids, P-42), prefixes having retained names or names systematically formed (see P-67.1.4.1) are used to denote inorganic acids.
Examples:
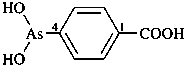
(HO)2P(O)-CH2-COOH
phosphonoacetic acid (PIN)
[2-(methoxysulfonyl)phenyl]phosphonic acid (PIN)
(an acid is senior to an ester)
(4-arsonobutyl)phosphonic acid (PIN)
(a phosphorus acid is senior to an arsenic acid)
C6H5-SO2-N=P(NH-C6H5)3
N-(trianilino-λ5-phosphanylidene)benzenesulfonamide (PIN)
N-(trianilinophosphoranylidene)benzenesulfonamide
[not N,N′,N′′-triphenyl-N′′′-benzenesulfonylphosphorimidic triamide;
nor N′′′-benzenesulfonylphosphorimidic tris(phenylamide)]
Examples:
Br2P(O)-CH2-CH2-P(S)Cl2
(2-phosphorodichloridothioylethyl)phosphonic dibromide (PIN)
(phosphonic dibromide is preferred to phosphonothioic dichloride;
O > S is considered before Cl > Br)
Cl2P(O)-O-CH2-CH2-O-P(O)Cl(NH2)
2-(phosphoramidochloridoyloxy)ethyl phosphorodichloridate (PIN)
(phosphorodichloridic acid preferred to phosphoramidochloridic acid)
aci-Nitro compounds deserve a special mention. They are tautomers of nitro compounds having the general structure R=N(O)OH or R2N(O)OH and are named as derivatives of azinic acid, H2N(O)OH.
Example:
The name ‘hydroxy(oxo)-λ5-azanylidene’ is a change from the 1993 (ref. 2) recommended name ‘hydroxynitroryl’, that is not acceptable in the context of these recommendations where two free valencies must be expressed by the correct ‘ylidene’ or ‘diyl’ type.
Examples:
4-[hydroxy(oxo)-λ5-azanylidene]cyclohexane-1-carboxylic acid (PIN)
4-aci-nitrocyclohexane-1-carboxylic acid
Like mononuclear acids, polynuclear acids have retained names that are used as preselected names. Substitutive or additive names are not recommended. Retained names are used as parents and are modifiable by functional replacement in the same manner that mononuclear acids are, except that only prefixes are used for the functional replacement operation.
Di- and polynuclear noncarbon oxoacids, whose central atoms are B, P, As, Sb, S, Se, are described here. They are divided into three types. Di- and trinuclear acids are exemplified for each central atom. In some cases higher polynuclear acids are known. Their names are formed by using the appropriate multiplying prefixes to indicate the number of central atoms. Di- and polynuclear noncarbon oxoacids are discussed in Sections P-67.2 and P-67.3.
Insofar as their structures are known and conform to those of the phosphorus acids, arsenic and antimony acids are named in the same way as those of phosphorus, with ‘ars’ and ‘stib’, respectively, in place of ‘phosph’. Similarly, tellurium acids are named in the same way as those of selenium, by changing ‘selen’ to ‘tellur’ in the names of acids.
P-67.2.1 Preselected namesP-67.2.1 Preselected names
P-67.2.2 Functional replacement derivatives of di- and polynuclear noncarbon oxoacids
P-67.2.3 Acid halides and pseudohalides of di- and polynuclear noncarbon oxoacids
P-67.2.4 Amides and hydrazides of di- and polynuclear noncarbon oxoacids
P-67.2.5 Esters and anhydrides of di- and polynuclear noncarbon oxoacids
P-67.2.6 Substituent groups derived from polyacids
The following traditional names are retained as preselected names (for consistency in the names of polynuclear oxoacids, the numerical infix ‘di’ has been uniformly used in naming dinuclear ‘hypo’ acids). Although the ‘meta’ acids are for general nomenclature only, they are preselected names if the structure is unknown.
For consistency in the names of polynuclear oxoacids, the numerical infix ‘di’ has been uniformly used in naming dinuclear ‘hypo’ acids, for example, hypodiphosphorous acid, rather than hypophosphorous acid.
(HO)2B-B(OH)2
hypodiboric acid (preselected name)
(HO)3Si-O-Si(OH)3
disilicic acid (preselected name)
(HO)HP(O)-O-HP(O)(OH)
diphosphonic acid (preselected name)
(HO)(O)HP-PH(O)(OH)
hypodiphosphonic acid (preselected name)
HO-PH-O-PH-OH
diphosphonous acid (preselected name)
(HO)HP-PH(OH)
hypodiphosphonous acid (preselected name)
(HO)2P(O)-O-P(O)(OH)2
diphosphoric acid (preselected name)
(HO)2(O)P-P(O)(OH)2
hypodiphosphoric acid (preselected name)
(HO)2P-O-P(OH)2
diphosphorous acid (preselected name)
(HO)2P-P(OH)2
hypodiphosphorous acid (preselected name)
(HO)HAs(O)-O-HAs(O)(OH)
diarsonic acid (preselected name)
(HO)(O)HAs-HAs(O)(OH)
hypodiarsonic acid (preselected name)
HO-AsH-O-AsH-OH
diarsonous acid (preselected name)
(HO)HAs-AsH(OH)
hypodiarsonous acid (preselected name)
(HO)2As(O)-O-As(O)(OH)2
diarsoric acid (preselected name)
diarsenic acid
(HO)2(O)As-As(O)(OH)2
hypodiarsoric acid (preselected name)
hypodiarsenic acid
(HO)2As-O-As(OH)2
diarsorous acid (preselected name)
diarsenous acid
(HO)2As-As(OH)2
hypodiarsorous acid (preselected name)
hypodiarsenous acid
(HO)HSb(O)-O-HSb(O)(OH)
distibonic acid (preselected name)
(HO)(O)HSb-HSb(O)(OH)
hypodistibonic acid (preselected name)
HO-SbH-O-SbH-OH
distibonous acid (preselected name)
(HO)HSb-SbH(OH)
hypodistibonous acid (preselected name)
(HO)2Sb(O)-O-Sb(O)(OH)2
distiboric acid (preselected name)
(HO)2(O)Sb-Sb(O)(OH)2
hypodistiboric acid (preselected name)
(HO)2Sb-O-Sb(OH)2
distiborous acid (preselected name)
(HO)2Sb-Sb(OH)2
hypodistiborous acid (preselected name)
HO-SO2-O-SO2-OH
disulfuric acid (preselected name)
HO-SO2-SO2-OH
dithionic acid (preselected name)
hypodisulfuric acid
HO-SO-SO-OH
dithionous acid (preselected name)
hypodisulfurous acid
[HAsO3]n = (-As(O)(OH)O-)n
metaarsoric acid
(only for general nomenclature)
metaarsenic acid
[HAsO2]n = (-As(OH)O-)n
metaarsorous acid
(only for general nomenclature)
metaarsenous aicd
[HBO]n = (-B(OH)O-)n
metaboric acid
(only for general nomenclature)
[HPO3]n = (-P(O)(OH)O-)n
metaphosphoric acid
(only for general nomenclature)
[HPO2]n = (-P(OH)O-)n
metaphosphorous acid
(only for general nomenclature)
[H2SiO3]n = (-Si(OH)2O-)n
metasilicic acid
(only for general nomenclature)
[HSbO3]n = (-Sb(O)(OH)O-)n
metastiboric acid
(only for general nomenclature)
[HSbO2]n = (-Sb(OH)O-)n
metastiborous acid
(only for general nomenclature)
(HO)HP(O)-O-HP(O)-O-HP(O)(OH)
triphosphonic acid (preselected name)
(HO)2P(O)-O-P(O)(OH)-O-P(O)(OH)2
triphosphoric acid (preselected name)
HO-SO2-O-SO2-O-SO2-OH
trisulfuric acid (preselected name)
P-67.2.2.1 General methodologyP-67.2.2.1 General methodology
P-67.2.2.2 Replacement by –OO–, –S–, =S, –Se–, =Se, –Te–, =Te, –NH–, and =NH
Prefixes are used to indicate functional replacement of polynuclear noncarbon oxoacids, listed in P-67.1.2.3.3 and P-67.1.2.3.4. The prefixes are cited and numbered in alphabetical order in front of the retained name of the polyacid, with appropriate locants as required. Each acid is numbered from one end to the other, starting from and finishing at a central atom. Before applying the functional replacement operation, the principal function must be selected; it receives the lower locant. The order of functions to be considered is: acids (see P-67.2.2.2), acid halides and pseudohalides (see P-67.2.3), amides and hydrazides (see P-67.2.4).
P-67.2.2.2 Replacement by –OO–, –S–, =S, –Se–, =Se, –Te–, =Te, –NH–, and =NH
Functional replacement of oxygen atom(s) is denoted by prefixes, i.e., peroxy, for –OO–; thio, for –S– or =S; seleno, for –Se– or =Se; telluro, for –Te– or =Te; and imido, for –NH– or =NH. Superscripted italic letter locants N1 N2 are used to designate substitution on imido groups that are not linkages between atoms that receive numerical locants as part of the chain. For rules on primes, see P-16.9.
The use of superscripted ‘N’ locants is a change from previous recommendations serially letters for example, ‘N′’, ‘N′′’, etc. ; the priming serially follows the arabic numbers of the chain, the number of primes parallels the increasing value of the arabic numbers.
Superscripted italic letters are used to specifically locate the chalcogen atoms in acid groups, for example S3.
This is a change from previous recommendations where the specific location of a chalcogen atom in acid groups was described by an on line arabic number prefixed to the atomic symbol.
Examples:
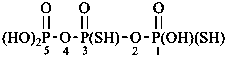
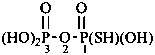
1-thiodiphosphoric S1-acid (name derived from the preselected name diphosphoric acid;
the ‘thio’ replacement determines locant ‘1’)
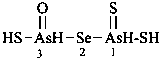
2-seleno-1,1,3-trithiodiarsonic S1,S3-acid
(name derived from the preselected name diarsonic acid;
the ‘dithio’ replacement for one acid group determines the locant ‘1’)
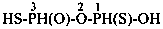
1,3-dithiodiphosphonic O1,S3-acid
(name derived from the preselected name diphosphonic acid;
the ‘OH’ group of the acid function is alphabetically preferred for lower locant to the ‘SH’ group)
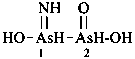
1-imidohypodiarsonic acid
(name derived from the preselected name hypodiarsonic acid;
the ‘imido’ replacement prefix determines the locant ‘1’)
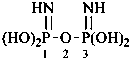
1,3-diimidodiphosphoric acid
(name derived from the preselected name diphosphoric acid)
1-thiodisulfuric S1-acid
(name derived from preselected name disulfuric acid)
3-amido-1-selenodisulfuric Se1-acid
(name derived from the preselected name disulfuric acid;
the acid determines the locant ‘1’)
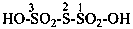
2-thiodisulfuric acid (preselected name)
sulfanedisulfonic acid
trithionic acid (traditional name)
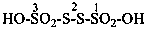
2-(dithioperoxy)disulfuric acid (preselected name)
disulfanedisulfonic acid
tetrathionic acid (traditional name)
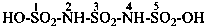
2,4-diimidotrisulfuric acid
(name derived from the preselected name trisulfuric acid)
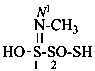
N1-methyl-1-imido-2-thiodithionous S2-acid (PIN;
the ‘imido’ replacememt prefix determines the locant ‘1’
[not 1-(methylimido)-2-thiodithionous 2-S-acid]
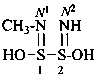
N1-methyl-1,2-diimidodithionous acid (PIN)
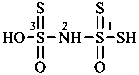
2-imido-1,1,3-trithiodisulfuric O3,S1-acid
(name derived from the preselected name disulfuric acid)
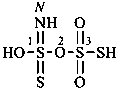
1-imido-1,3-dithiodisulfuric O1,S3-acid
(name derived from the preselected name disulfuric acid;
the ‘OH’ group of the acid function is alphabetically preferred for lower locant to the ‘SH’ group)
Acid halides and pseudohalides, in which all OH groups have been replaced by halides or pseudohalide atoms or groups, are named by functional class nomenclature by replacing the name ‘acid’ by the name of the appropriate halide or pseudohalide. For acid halides and acid pseudohalides the senority is halides (alphabetical) followed by the following seniority for the halogenides: N3, CN, NC, NCO, NCS, NCSe, NCTe.
Examples:
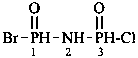
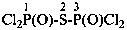
2-thiodiphosphoric tetrachloride
(name derived from the preselected name diphosphoric acid)
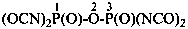
diphosphoric tetraisocyanate (PIN)
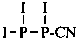
cyanohypodiphosphorous triiodide (PIN)
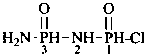
3-amido-2-imidodiphosphonic chloride
(name derived from the preselected name diphosphonic acid,
the acid chloride is preferred to the amide group and determines the locant ‘1’)
Polynuclear noncarbon oxoacids in which all –OH groups have been replaced by –NH2 or –NHNH2 groups are named as amides or hydrazides, respectively, by functional class nomenclature. Amides are expressed by the class name ‘amide’, preceded by an appropriate multiplying prefix, ‘di’, ‘tri’, etc. Similarly, hydrazides are expressed by the class name ‘hydrazide’. Functional replacement is described by prefixes cited before the whole name and not in front of the class name. Italic letter locants N, N′, etc. and superscripted letter locants N1, N′1, etc. are used to designate substitution on nitrogen atoms that are not linkages between central atoms that receive numerical locants as part of the chain. When a choice is needed, amides are senior to hydrazides, and receive the lower locants.
Examples:
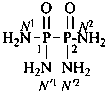
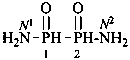
hypodiphosphonic diamide
(name derived from the preselected name hypodiphosphonic acid)
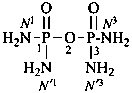
diphosphoric tetraamide
(name derived from the preselected name diphosphoric acid)
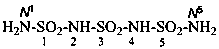
2,4-diimidotrisulfuric diamide
(name derived from the preselected name trisulfuric acid)
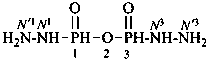
diphosphonic dihydrazide
(name derived from the preselected name diphosphonic acid)
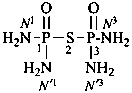
2-thiodiphosphoric tetraamide
(name derived from the preselected name diphosphoric acid)
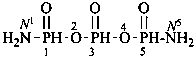
triphosphonic diamide
(name derived from the preselected name triphosphonic acid)
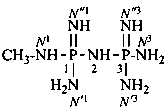
N1-methyl-1,2,3-triimidodiphosphoric tetraamide (PIN)
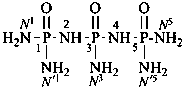
2,4-diimidotriphosphoric pentaamide
(name derived from the preselected name triphosphoric acid)
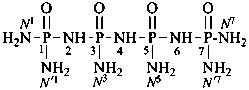
2,4,6-triimidotetraphosphoric hexaamide
(name derived from the preselected name tetraphosphoric acid)
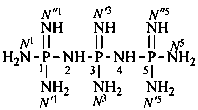
pentaimidotriphosphoric pentaamide
(name derived from the preselected name triphosphoric acid)
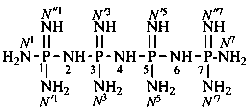
heptaimidotetraphosphoric hexaamide
(name derived from the preselected name tetraphosphoric acid)
H2N-SO2-O-SO2-NH2
disulfuric diamide
(name derived from the preselected name disulfuric acid)
H2N-SeO2-O-SeO2-NH2
diselenic diamide
(name derived from the preselected name diselenic acid)
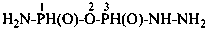
3-hydrazidodiphosphonic 1-amide
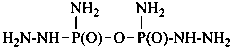
1,3-dihydrazidodiphosphoric 1,3-diamide
P-67.2.5.1 Salts of di- and polybasic noncarbon oxoacidsP-67.2.5.1 Salts of di- and polynuclear noncarbon oxoacids
P-67.2.5.2 Esters of polybasic noncarbon oxoacids
P-67.2.5.3 Anhydrides of polybasic noncarbon oxoacids
P-67.2.5.1.1 Neutral salts of di- and polynuclear noncarbon oxoacids are named by citing the cation(s) followed by the name of the anion(s) as (a) separate word(s). Names of anions are formed by changing the ‘ic acid’ ending to ‘ate’ and the ‘ous acid’ to ‘ite’. Different cations are cited in alphabetical order.
Examples:
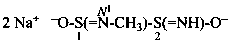
4 Na+ (–O)2P(=O)-O-P(=O)(O–)2
tetrasodium diphosphate
(name derived from the preselected name diphosphoric acid)
Example:
Fully esterified polynuclear noncarbon oxoacids are named as neutral salts, except that names of allowed groups (alkyl groups, aryl groups, etc.), cited in alphanumerical order when more than one, replace the name of the cations. Partial (acid) esters of polybasic noncarbon oxoacids and their salts are named by the procedures for neutral esters and acid salts, except that the name ‘hydrogen’ denoting acid hydrogen atoms is indicated by the separate word ‘hydrogen’ (with the appropriate multiplying prefix denoting multiplicity) inserted between the name of the cation or of the organic group and the name of the anion.
Examples:
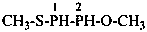
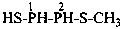
methyl hydrogen dithiohypodiphosphonite (PIN)
CH3-O-SO2-O-SO2-S-CH2-CH3
S-ethyl O-methyl thiodisulfate (PIN)
(CH3)2CH-O-SO-O-SO-O-CH(CH3)2
1,3-bis[(propan-2-yl)oxy]-1λ4,3λ4-dithioxane-1,3-dione (PIN)
[not di(propan-2-yl) disulfite, see P-67.3.2]
CH3-O-SO2-S-SO2-O-CH3
O1,O3-dimethyl thiodisulfate (PIN)
Neutral anhydrides formed between organic acids and polynuclear noncarbon oxoacids having preselected names are named by citing, in alphabetical order, the names of the acids followed by the name of the class ‘anhydride’; multiplying prefixes ‘di’, ‘tri’, etc. are used to indicate the multiplicity of the anhydride linkages.
Acidic anhydrides are named by using the senior acid as parent or by using systematic substitutive nomenclature as described in P-67.3.1.
Examples:
CH3-CO-O-SO-SO-O-CO-CH2-CH3
acetic dithionous propanoic dianhydride (PIN)
acetic hypodisulfurous propanoic dianhydride
In the presence of a characteristic group having precedence for citation as principal group, polynuclear noncarbon oxoacids are cited as prefixes. The names of these prefixes are formed by:
(1) combinations of acyl groups;When there is a choice for parent substituent, seniority is given to parent substituents having the largest size, then, if needed, to alphanumerical order.(2) on the basis of the names of the group which includes the greatest number of P, As, Sb, S, Se, and Te central atoms.
(3) skeletal replacement nomenclature (see P-15.4 and P-51.4), when conditions for its use are satisfied.
Examples:
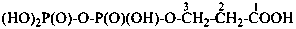
(3) 3,5,7-trioxo-2,4,6,8-tetraoxa-3λ4,5λ4,7λ4-trithiadecan-10-oic acid (PIN)
(1) {[({[(methoxysulfinyl)oxy]sulfinyl}oxy)sulfinyl]oxy}acetic acid
(2) [(5-methoxy-1,3,5-trioxo-1λ4,3λ4,5λ4-trisulfoxan-1-yl)oxy]acetic acid
(3) 3,5-bis(sulfanylidene)-2,3λ4,4,5λ4,6-pentathianonan-9-oic acid (PIN)
(2) 3-[5-methyl-2,4-bis(sulfanylidene)-2λ4,4λ4-pentasulfanyl]propanoic acid
(1) 3-[({[(methylsulfanyl)sulfinothioyl]sulfanyl}sulfinothioyl)sulfanyl]propanoic acid
P-67.3.1 Names of polynuclear noncarbon oxoacids that cannot be formed on the basis of fundamental oxoacids are either named by using class names, such as anhydrides, or they are formed substitutively. Anhydride names are preferred to substitutive names.
Some names are also included in this Section because their structure does not correspond to the name implied by a diacid or a hypodiacid, for example the name ‘disulfurous acid’, exemplified below.
Names of derivatives of acids that cannot be formed directly by functional replacement are generated by substitution of acids with preferred names such as phosphonic acid and phosphinic acid; or they are class names such as ‘anhydride’. The parent is chosen in accordance with the seniority order of classes: acids, acid halides, azides, cyanides, isocyanides, isocyanates (and chalcogen analogues in the order O > S > Se > Te), amides, hydrazides, and the maximum number of groups representing the senior class (see P-67.1.2.3.2).
Examples:
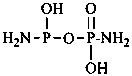
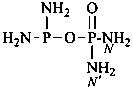
phosphorodiamidic phosphorodiamidous anhydride (preselected name)
[(diaminophosphanyl)oxy]phosphonic diamide
(an anhydride is senior to an amide)
(arsonosulfanyl)phosphonic acid (preselected name)
arsoric phosphoric thiomonoanhydride
(an acid is senior to an anhydride; the phosphorus acid is senior to the arsenic acid)
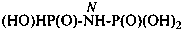
N-(hydroxyphosphonoyl)phosphoramidic acid (preselected name)
(only a substitutive name is possible in this case)
(HO)2P-HP(O)-OH
(dihydroxyphosphanyl)phosphinic acid (preselected name)
(CH3-O)2P(O)-O-HP(O)-O-CH3
methyl [(dimethoxyphosphoryl)oxy]phosphonate (PIN)
(phosphonic acid is senior to phosphoric acid; see P-42)
(formerly trimethyl isohypophosphate)
(CH3)2P-P(OH)-P(CH3)2
bis(dimethylphosphanyl)phosphinous acid (PIN)
(not 1,1,3,3-tetramethyltriphosphan-2-ol;
the acid is senior to the heterol)
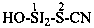
1-hydroxy-1,1-diiodo-1λ4-disulfane-2-carbonitrile (PIN)
(formerly diiodo(thiocyanatido)orthosulfurous acid)
(not orthosulfurodiiodidothiocyanatidous acid)
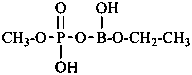
methyl hydrogen {[ethoxy(hydroxy)boranyl]oxy}phosphonate (PIN)
(ethyl dihydrogen borate) (methyl dihydrogen phosphate) anhydride
CH3-CO-O-P(O)(OH)2
(acetyloxy)phosphonic acid (PIN)
acetic phosphoric monoanhydride
monoacetyl phosphate
HO-SeO2-O-SO2-OH
selenic sulfuric anhydride (preselected name)
(selenonooxy)hydroxy-λ6-sulfanedione
CH3-CH2-CO-O-B(OH)2
(propanoyloxy)boronic acid (PIN)
boric propanoic monoanhydride
CH3-CO-O-CO-O-CO-OH
{[(acetyloxy)carbonyl]oxy}formic acid (PIN)
acetic dicarbonic monoanhydride
The name disulfurous acid should correspond to the structure HO-SO-O-SO-OH implied for all homogeneous diacids in accordance with the definition given in P-67.2. As the reported structure, HO-SO-SO2-OH, is called ‘disulfurous acid’ in the Nomenclature of Inorganic Chemistry (ref. 12) a different name must be assigned to HO-SO-O-SO-OH. A substitutive name is appropriate in this situation.
Since the name ‘disulfurous acid’ is used for HO-SO-SO2-OH in the Nomenclature of Inorganic Chemistry ref. 12), it cannot be used in the systematic way for HO-SO-O-SO-OH. Accordingly, the preselected name for the latter is ‘1,3-dihydroxy-1λ4,3λ4-dithioxane-1,3-dione’.
Examples:
CH3-O-S(=O)-O-S(=O)-O-CH3
1,3-dimethoxy-1λ4,3λ4-dithioxane-1,3-dione (PIN)
CH3-O-S(=NH)-O-S(=NH)-O-CH3
1,3-dimethoxy-1λ4,3λ4-dithioxane-1,3-diimine (PIN)
P-68.0 IntroductionP-68.0 INTRODUCTION
P-68.1 Nomenclature for compounds of the Group 13 elements
P-68.2 Nomenclature for compounds of the Group 14 elements
P-68.3 Nomenclature for compounds of the Group 15 elements
P-68.4 Nomenclature for compounds of the Group 16 elements
P-68.5 Nomenclature for compounds of the Group 17 elements
The nomenclature of organic compounds is based on two approaches. General principles, rules, and conventions applicable, with some rare exceptions, to all compounds of elements belonging to Groups 13 through 17; they have been described and illustrated in previous Chapters. Another approach is based on the identical treatment of compounds within one Group. Up until now the nomenclature of compounds of Group 13 was compounds of the element boron; these will be included in a future publication by another group. It is advantageous to describe all compounds of a Group in the same manner to identify clearly their similarities and underline exceptions, if any. This method should facilitate naming of new compounds by comparison with established models.
Another aspect of nomenclature that is described Group by Group would be to easily grasp the general patterns of the different Groups. The nomenclature of Group 15 is diverse; suffixes are associated with nitrogen, mononuclear and polynuclear acids are the base of many derivatives of phosphorus, arsenic, and many compounds of antimony and bismuth compounds are named substitutively. By comparison, compounds of Group 14 are essentially named by substitutive nomenclature. Thus, the compound C6H5-PH-OCH3 is named by functional class nomenclature as an ester, i.e., methyl phenylphosphinite, but the compound, C6H5-SnH(OCH3)2 is named substitutively as dimethoxy(phenyl)stannane in spite of their apparent similarity of structures.
There is a third purpose for this Section about Groups 13 through 17. In it, substitutive nomenclature treats metals, semi-metals, and non-metals equally when a compound contains carbon. The Group treatment illustrates many aspects of the nomenclature of organometallic compounds. This is particularly evident for the nomenclature of compounds of the Groups 13 and 14 elements, for which the extensive and well known nomenclature for boron and carbon compounds serves as models to name the other compounds of the Group.
For boron and the metals of Groups 14 and 15, i.e., Ge, Sn, Pb, Sb, and Bi, the same principles are followed as in other sections of these recommendations, namely, if the compound contains carbon and can be named by substitutive nomenclature principles given elsewhere in these recommendations based on acceptable parent hydride names, it is assigned a PIN; however, if there is no carbon present, the name is described as a preselected parent hydride or a name based on a preselected parent hydride or compound. Finally, for many compounds of these elements, their structures are not consistent with the principles of substitutive nomenclature; these, then, are named by the principles of inorganic and coordination nomenclature, for which see ref. 12.
P-68.1 NOMENCLATURE FOR COMPOUNDS OF THE GROUP 13 ELEMENTS
Except for the polynuclear boron hydrides, boron compounds have traditionally been included in the recommendations for naming organic compounds. However, substitutive nomenclature has been applied on the basis of parent hydrides, their substituent groups, and appropriate operations to complement the nomenclature of polynuclear boron compounds, for example names formed by skeletal replacement (‘a’) nomenclature, multiplicative nomenclature, and names derived from the functional parent acids based on boric acid, B(OH)3, boronic acid, HB(OH)2, and borinic acid, H2B(OH); these are retained functional parent compounds.
The nomenclature of Al, Ga, In, and Tl compounds in these recommendations, including formal organometallic compounds, is patterned on boron nomenclature and is recommended only for use in general nomenclature. Recommendations of preferred IUPAC names for most compounds of these elements will appear in a later publication by another group.
P-68.1.1 Parent hydridesP-68.1.1 Parent hydrides
P-68.1.2 Names for substituent groups derived from parent hydrides
P-68.1.3 Modification of the degree of hydrogenation
P-68.1.4 Functional parent compounds
P-68.1.5 Substitutive nomenclature
P-68.1.6 Adducts
P-68.1.1.1 Mononuclear hydridesP-68.1.1.1 Mononuclear hydrides
P-68.1.1.2 Acyclic polynuclear hydrides
P-68.1.1.3 Cyclic hydrides
The names of the mononuclear hydrides are listed in Table 2.1. The standard bonding number is 3 and the λ-convention (see P-14.1) is used to indicate nonstandard bonding numbers. The names of these parent hydrides are all preselected names (see P-12.2).
Examples:
BH
λ1-borane (preselected name)
AlH3
alumane (preselected name)
GaH3
gallane (preselected name)
InH3
indigane (preselected name)
TlH3
thallane (preselected name)
P-68.1.1.2.1 Acyclic di- and polynuclear parent hydrides are named by citing the number of skeletal atoms in the molecule as a multiplying prefix, ‘di’, ‘tri’, etc., in front of the name of the mononuclear parent hydride. The number of hydrogen atoms in the molecule is designated by an arabic numeral enclosed in parentheses immediately following the name derived as above. These numbers are omitted when there is no ambiguity or by convention in the names of acyclic polynuclear hydrides. A specific nomenclature is used to name polycyclic polyboranes; these names are described in Chapter IR-6.2.3 of ref. 12. Parent hydrides are preselected names.
Examples:
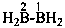
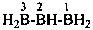
triborane(5) (preselected name)
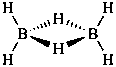
diborane(6) (preselected name)
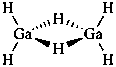
digallane(6) (preselected name)
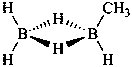
1-methyldiborane(6) (PIN)
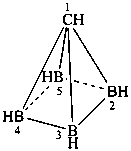
1-carba-nido-pentaborane(5) (PIN)
When nitrogen atoms are present, this is a change from the 1993 Guide (ref. 2) because the ‘amine’ characteristic group was not recognized there.
Examples:
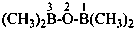
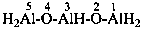
trialuminoxane (preselected name)
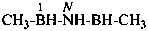
1-methyl-N-(methylboranyl)boranamine (PIN)
Examples:
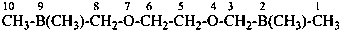
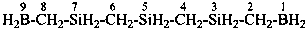
3,5,7-trisila-1,9-diboranonane (PIN)
[silanediylbis(methylene)]bis[(boranylmethyl)silane]
Si,Si′-bis(boranylmethyl)[silanediylbis(methylene)]bis(silane)
P-68.1.1.3.1 Polyhedral polyboranes.
Nomenclature of polyhedral polyboranes constitute a rich and diversified system characterized by specific prefixes. It has been described and illustrated in the Section IR-6.2.3 (ref. 12); it is not reproduced in these recommendations.
Examples:
nido-pentaborane(9)
(preselected name)
1,2-dicarba-closo-decaborane(10) (PIN)
Examples:
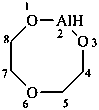
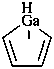
1H-gallole
(not 1-gallacyclopenta-2,4-diene)
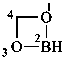
1,3,2-dioxaboretane (PIN)
(not 1,3-dioxa-2-boracyclobutane)
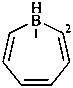
1H-borepine (PIN)
(not 1H-1-boracyclohepta-2,4,6-triene)
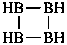
tetraboretane (preselected name; see P-12.2; P-22.2.5)
cyclotetraborane(4)
(not 1,2,3,4-tetraboracyclobutane)
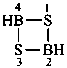
1,3,2,4-dithiadiboretane (preselected name; see P-12.2; P-22.2.6)
cyclodiborathiane
(not 1,3-dithia-2,4-diboracyclobutane)
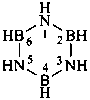
1,3,5,2,4,6-triazatriborinane
(preselected name) borazine (see D-7.54, ref. 1)
cyclotriborazane
(not 1,3,5-triaza-2,4,6-triboracyclohexane)
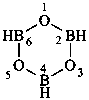
1,3,5,2,4,6-trioxatriborinane (preselected name)
boroxin (see D-7.54, ref. 1)
cyclotriboroxane
(not 1,3,5-trioxa-2,4,6-triboracyclohexane)
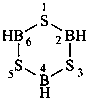
1,3,5,2,4,6-trithiatriborinane (preselected name)
borthiin (see D-7.54, ref. 1)
cyclotriborathiane
(not 1,3,5-trithia-2,4,6-triboracyclohexane)
Examples:
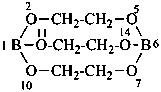
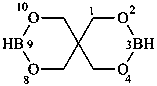
2,4,8,10-tetraoxa-3,9-diboraspiro[5.5]undecane (PIN)
Examples:
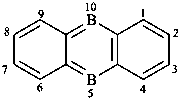
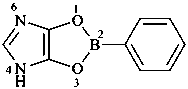
2-phenyl-2H,4H-[1,3,2]dioxaborolo[4,5-d]imidazole (PIN)
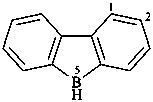
5H-dibenzo[b,d]borole (PIN)
Names of substituent groups –BH2 and =BH derived from borane are formed by method (2) described in P-29.2; they are preselected prefixes.
Prefixes derived from borane are no longer named by method (1) in P-29.2.
Examples:
=BH
boranylidene (preselected prefix)
(not borylidene)
>BH
boranediyl (preselected prefix)
(not borylidene)
(not borylene)
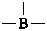
boranetriyl (preselected prefix)
(not borylidyne)
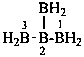
2-boranyltriborane(5) (preselected prefix)
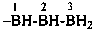
triboran(5)-1-yl (preselected prefix)
–BH-O-BH2
diboroxanyl (preselected prefix)
–BH-NH-BH2
(boranylamino)boranyl (preselected prefix)
(not diborazan-1-yl)
H2Al–
alumanyl
(preselected prefix)
H2Ga–
gallanyl
(preselected prefix)
H2In–
indiganyl
(preselected prefix)
H2Tl–
thallanyl
(preselected prefix)
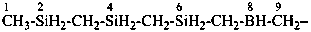
2,4,6-trisila-8-boranonan-9-yl (preferred prefix)
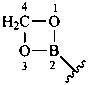
1,3,2-dioxaboretan-2-yl (preferred prefix)
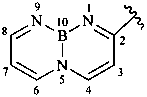
[1,3,2]diazaborinino[1,2-a][1,3,2]diazaborinin-2-yl (preferred prefix)
Double bonds are denoted by the ‘ene’ ending or by saturation of double bonds of mancude ring systems by the use of ‘hydro’ prefixes, as described in Sections P-31.1 and P-31.2, respectively.
Hydro/dehydro prefixes are now classified as detachable prefixes but are not included in the category of alphabetized detachable prefixes (see P-14.4; see also P-15.1.5.2, P-31.2, P-58.2).
Examples:
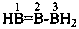
1-methyldecahydro-1-benzaluminine
P-68.1.4.1 The names boric acid, boronic acid, and borinic acid are preselected retained names for the compounds B(OH)3, HB(OH)2, and H2B(OH), respectively (see P-67.1.1). They are preferred IUPAC names for deriving the names of the corresponding salts, esters, or anhydrides; or when the hydrogen atom(s) attached to the boron atom is (are) substituted, for example methylboronic acid for CH3-B(OH)2. Chalcogen analogues are named using infixes to denote functional replacement of oxygen by S, Se, and Te.
Acid names such as these are not used for other elements of Group 13, which are named using substitutive nomenclature based on the appropriate parent hydride. For names such as methaneboronic acid vs. methylboronic acid for CH3-B(OH)2, see P-67.1.1.2.
Examples:
CH3-B(O–)(OH) Na+
sodium hydrogen methylboronate (PIN)
CH3-B(OH)2
methylboronic acid (PIN)
(not methylboranediol)
(CH3)2B(SH)
dimethylborinothioic acid (PIN)
dimethyl(thioborinic) acid
(not dimethylboranethiol)
B(S-CH3)3
trimethyl borotrithioate (PIN)
trimethyl trithioborate
S-ethyl O-methyl hydrogen borothioate (PIN)
S-ethyl O-methyl hydrogen thioborate
ethylboronochloridic acid (PIN)
ethylchloroboronic acid; chloro replaces an OH group of boronic acid)
chloro(ethyl)borinic acid
(chloro and ethyl
substitute the H atoms of borinic acid)
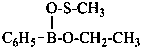
O-ethyl OS-methyl phenylborono(thioperoxoate) (PIN)
O-ethyl OS-methyl phenyl(thioperoxy)boronate
(CH3)2Al-O– Na+
sodium dimethylalumanolate
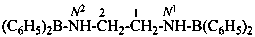
N1,N2-bis(diphenylboranyl)ethane-1,2-diamine (PIN)
[not N,N′-(ethane-1,2-diyl)bis(diphenylborinic amide);
not N,N′-(ethane-1,2-diyl)bis(diphenylboranamine);
not 1,1,6,6-tetraphenyl-2,5-diaza-1,6-diborahexane]
The general methodology for forming substituent groups derived from the boron acids has been described in P-67.1.4.2. Except for the name ‘borono’ for (HO)2B– which is retained, their names are formed substitutively based on the parent hydride ‘borane’, BH3. Chalcogen analogues of ‘borono’ are named by replacement prefixes, and not replacement infixes.
Examples:
(HS)BH–
sulfanylboranyl (preselected prefix)
(HO)(HS)B–
thioborono (preselected prefix)
hydroxy(sulfanyl)boranyl
(HSe)2B–
diselenoborono (preselected prefix)
bis(selanyl)boranyl
Cl-BH–
chloroboranyl (preselected prefix)
(not chloroboryl)
(H2N)2B–
diaminoboranyl (preselected prefix)
(CH3)2B-O–
(dimethylboranyl)oxy (preferred prefix)
CH3-BH-NH–
(methylboranyl)amino (preferred prefix)
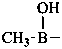
hydroxy(methyl)boranyl (preferred prefix)
Derivatives of borane, BH3, are named by substitutive nomenclature, the substituents being denoted by suffixes and prefixes in accordance with the principles, rules, and conventions of substitutive nomenclature. The three acids described in P-68.1.4 have retained names that are preselected names.
Derivatives are named in accordance with the seniority of classes described by the general rule in Section P-41. Thus, acids having retained names have seniority over other suffixes. Suffixes are used when recommended in substitutive nomenclature, in accordance with the general rule described in Section P-43. In the absence of a suffix that has priority for naming organic compounds, when a choice is possible for selecting the parent hydride, the seniority order is as follows: N > P > As > Sb > Bi > Si > Ge > Sn > Pb > B > Al > Ga > In > Tl > O > S > Se > Te > C (substituent groups).
P-68.1.5.1 Suffix nomenclatureP-68.1.5.1 Suffix nomenclature
P-68.1.5.2 Prefix nomenclature
Suffixes, when available, are used to denote characteristic groups; prefixes are not recommended. Suffixes containing a carbon atom generate preferred IUPAC names.
Examples:
(HO)2B-B(OH)2
hypodiboric acid (preselected name; see P-67.2.1)
diborane(4)tetrol
[not tetrahydroxydiborane(4)]
(CH3)2Tl-OH
dimethylthallanol
hydroxydi(methyl)thallane
(CH3)2Tl-O– Na+
sodium dimethylthallanolate
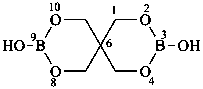
2,4,8,10-tetraoxa-3,9-diboraspiro[5.5]undecane-3,9-diol (PIN)
P-68.1.5.2.1 Substituted parent hydridesP-68.1.5.2.1 Substituted parent hydrides
P-68.1.5.2.2 Compounds with bridging atoms or groups
P-68.1.5.2.3 Compounds with groups of higher seniority
Normal prefix names are used to describe substituents of the B, Al, In, or Tl parent hydrides.
Examples:
Al(C2H5)3
triethylalumane
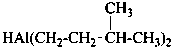
bis(3-methylbutyl)alumane
Al(O-CH2-CH2-CH2-CH3)3
tributoxyalumane
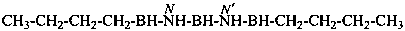
N,N′-bis(butylboranyl)boranediamine (PIN)
(not 1,5-dibutyltriborazane)
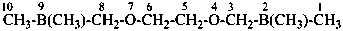
2,9-dimethyl-4,7-dioxa-2,9-diboradecane (PIN)
[ethane-1,2-diylbis(oxymethylene)]bis(dimethylborane)
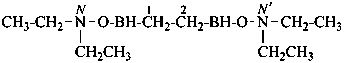
N,N′-[ethane-1,2-diylbis(boranediyloxy)]bis(N-ethylethanamine) (PIN)
O,O′-[ethane-1,2-diylbis(boranediyl)]bis(N,N-diethylhydroxylamine)
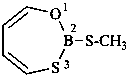
2-(methylsulfanyl)-2H-1,3,2-oxathiaborepine (PIN)
4,5-diethyl-2,2,3-trimethyl-2,5,7,8-tetrahydro-1,6,2,5-dioxasilaborocine (PIN)
1-ethyl-2,3-diphenyl-1H-aluminirene
Derivatives of di- and polyheteranes of the Group 13 elements, such as diborane, polyboranes, and related Al, Ga, In, and Tl congeners, are named using substitutive nomenclature. When a nonbridging hydrogen is substituted, locants, including the locant ‘1’, are used in the customary way. Bridging atoms or groups are indicated as follows:
(a) a bridging substituent is indicated by adding the Greek letter μ (mu) immediately before the name of the substituent and separating its prefix name from that of the rest of the name by hyphens;where the same substituent is present as a bridging group and as a nonbridging substituent it is cited first as a bridging substituent.(b) two or more bridging substituents of the same kind are indicated by ‘di-μ’ or ‘bis-μ’, and so on;
(c) bridging substituents are listed with the other substituents in alphanumerical order;
Examples:
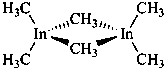
di-μ-methyl-tetramethyldiindigane(6)
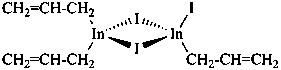
di-μ-iodo-1-iodo-1,2,2-tris(prop-2-en-1-yl)diindigane(6)
P-68.1.5.2.3 Compounds with groups of higher seniority
In the presence of groups of higher seniority, parent structures and prefixes are chosen in accordance with the seniority of classes (see P-41).
Examples:
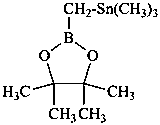
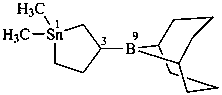
3-(9-borabicyclo[3.3.1]nonan-9-yl)-1,1-dimethylstannolane (PIN)
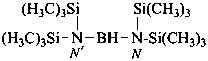
N,N,N′,N′-tetrakis(trimethylsilyl)boranediamine (PIN)
[not N,N′-boranediylbis[1,1,1-trimethyl-N-(trimethylsilyl)silanamine];
the diamine is senior to a monoamine even though ‘Si’ is senior to ‘B’ ( see P-44.1.1)
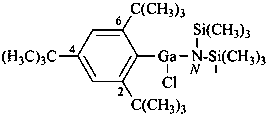
N-[chloro(2,4,6-tri-tert-butylphenyl)gallanyl]-1,1,1-trimethyl-N-(trimethylsilyl)silanamine
(Si is senior to Ga)
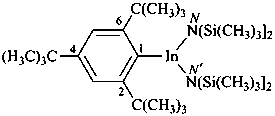
[(2,4,6-tri-tert-butylphenyl)-N,N,N′,N′-tetrakis(trimethylsilyl)indiganediamine
{not N,N′-[(2,4,6-tri-tert-butylphenyl)indiganediyl]bis[1,1,1-trimethyl-N-(trimethylsilyl)silanamine];
the diamine is senior to a monoamine even though Si is senior to In; see P-44.1.1}
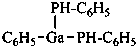
P,P′-(phenylgallanediyl)bis(phenylphosphane)
(P is senior to Ga)
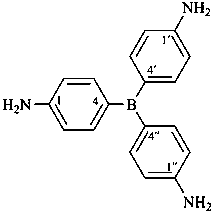
4,4′,4′′-boranetriyltrianiline (PIN; a multiplicative name)
4,4′,4′′-boranetriyltris(benzen-1-amine)
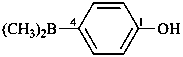
4-(dimethylboranyl)phenol (PIN)
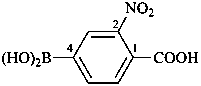
4-borono-2-nitrobenzoic acid (PIN)
(CH3)3Si-BF2
(difluoroboranyl)tri(methyl)silane (PIN)
(Si is senior to B)
[not (trimethylsilyl)boronic difluoride; see P-67.1.2.5.2]
Ga(S-S-CH2-CH3)3
tris(ethyldisulfanyl)gallane
tris[ethyl(dithioperoxy)]gallane
Al(O-CO-[CH2]16-CH3)3
alumanetriyl tri(octadecanoate)
(a pseudoester)
An adduct is a new chemical species (AB), each molecular entity of which is formed by direct combination (addition) of two separate molecular entities (A) and (B) in such a way that there may be a change of connectivity but no gain or loss of atoms in either of the molecular entities (see ref. 18). ‘Adduct’ is a general term specifically used for products of addition reactions which, whenever appropriate, should be used in preference to the less explicit term ‘complex’. Stoichiometries other than ‘1:1’ are also possible, e.g. ‘2:1’, a bis-adduct. An intramolecular adduct is formed when A and B are groups contained within the same molecular entity.
In this section Lewis adducts involving boron compounds are described based on the general principles for organic adducts given in P-14.8. The Lewis acid component is usually an organic boron compound, the electron-pair acceptor; the Lewis base component is usually, but not always, an organic nitrogen compound.
For adducts composed solely of organic compounds, the individual components are cited in the order of seniority of classes (see P-41) in formulas, no longer according to the number of species in the adduct, nor in accordance with the alphanumerical order as recommended in the 1979 Recommendations (see Rule D-1.55, ref. 1) and in the revised Nomenclature of Inorganic Chemistry, 2005 Recommendations (ref. 12).
P-68.1.6.1 Structures for Lewis ‘adducts’
These adducts may be separate species that are mixed at the molecular level or be attached to each other in an undefined way, in which case the components are associated with each other in the formula using a center dot. When the attachments are known, the components have been shown in three different ways, each of which has connotations to the type of nomenclature used.
One of the fundamental principles of organic chemical nomenclature is to name the structure that is drawn, and not to ascertain what an actual electronic configuration might be. Hence, one must be prepared to name any of the following structures that might in fact be drawn.
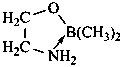
(II)
(III)
Structure (II) shows a connection using a covalent bond now favored to describe a bond consisting of two electrons regardless of their origin (ref. 18). It calls for a nomenclature using coordination principles, and would be the structures used in this document for the generation of coordination names when they are given, even though such structures are not shown. To use organic principles to name a structure showing a strict covalent bond would require use of the λ-convention for the atoms involved in the connection which simply is not favored for intermediate bonding numbers.
Structure (III) is the formal representation of structure (II) showing the formal charges on the involved atoms which would be required in order to use organic principles in naming this structure.
Organic nomenclature principles would require a zwitterionic name (see P-74), which tend to be largely avoided in organic nomenclature. No zwitterionic names are given in this subsection.
In the formulas of Lewis adducts, Lewis bases, which are generally organic in nature, are cited first following the seniority order of classes of organic compounds, rings and ring systems, and of chains as indicated in Chapter P-4, followed by inorganic compounds, i.e., compounds which do not contain carbon, in order of increasing number and, if they occur in equal numbers, in alphabetical order of the first symbols of the formulas. Lewis acids are then cited in the same order. Addition compounds containing water are exceptional, in that the water is always cited last.
P-68.1.6.2 General organic method for naming Lewis adducts
Adducts (addition compounds) between neutral Lewis bases and Lewis acids are named by citing the name of each component in the order given by the formula and connecting the name of each component by an ‘em’ dash (see P-16.2.4.5) or by the set of connected atomic symbols separated by an ‘em’ dash and enclosed in parentheses. The number of molecules of each component is denoted by two methods:
(1) appropriate multiplying prefixes, except for mono; orWhen desired, and especially when there may be more than one possible donor or acceptor atom, the attachments are indicated in the name by italicized atomic symbols joined by an ‘em’ dash (see P-16.2.4.5), enclosed in parentheses, and cited between the names of each component of the adduct. Each atomic symbol refers to the component nearest to it. The names of components and the atomic symbols are cited in the sequence donor-acceptor, the locant of a component being added before the appropriate atomic symbols, as required.(2) by indicating the proportions of the species, if necessary, after the complete name by arabic numbers separated by a solidus (/) and placed in parentheses with a space in front of the first parenthesis; water, if present, is cited last in the name, and the number of water molecules per formula unit is therefore also cited last among these numerals within the parentheses.
However, these ‘adduct names’ are not PINs; the latter are coordination or ‘inorganic’ names (see ref. 12). Thus, in this document, PINs for these adducts are not assigned. They will be assigned in a forthcoming document on PINs for inorganic and coordination compounds.
Examples:
(CH3)3N→BCl3
N,N-dimethylmethanamine—trichloroborane (1/1)
trichlorido(N,N-dimethylmethanamine-κN)boron
(CH3-CH2)2S→H2B-CH3
(ethylsulfanyl)ethane—methylborane (1/1)
[(ethylsulfanyl-κS)ethane]dihydridomethanidoboron
ethanamine(N→B2)(N→B4)pentaborane(9) (2/1)
2,4-bis(ethanamine-κN)-2,3:2,5:3,4:4,5-tetra-μH-nido-pentaborane(9)
ethane-1,2-diamine—borane (1/2)
ethane-1,2-diamine—bis(borane)
μ-(ethane-1,2-diamine-1κN1,2κN2)-bis(trihydridoboron)
(CH3)3N • (CH3)2S • B12H10
N,N-dimethylmethanamine–(methylsulfanyl)methane–dodecaborane(10) (1/1/1)
(trimethyl)amine–(methylsulfanyl)methane–dodecaborane(10) (1/1/1)
(N,N-dimethylmethanamine)decahydrido[(methylsulfanyl)methane]dodecaboron
H3C-O-NH2→BH3
O-methylhydroxylamine(N—B)borane (1/1)
trihydrido(O-methylhydroxylamine-κN)boron
(CH3)2NPF2 • B4H8
N,N-dimethylphosphoramidous difluoride(P—B)tetraborane(8) (1/1)
(N,N-dimethylphosphoramidous difluoride-κP)octahydridotetraboron
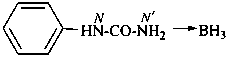
N-phenylurea(N′—B)borane (1/1)
trihydrido(N-phenylurea-κN′)boron
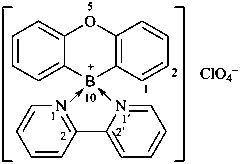
2,2′-bipyridine(N1,N1′—B)-10H-phenoxaborin-10-ylium perchlorate
(2,2′-bipyridine-κ2N1,N1′)[2,2′-oxybis(benzen-1-ido-κC1)]boron(1+) perchlorate
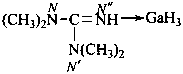
N,N,N′,N′-tetramethylguanidine(N′′—Ga)gallane (1/1)
trihydrido[N,N,N′,N′-tetramethylguanidine-κN′′]gallium
An intramolecular adduct between a group acting as a Lewis base and another group acting as a Lewis acid in the same molecule is denoted by atomic symbol pairs in the order donor-acceptor as described in Rule P-68.1.6.2, but enclosed in parentheses and cited in front of an appropriate portion of the name of the compound. A symbol denoting the proportions of the components is not required.
Examples:
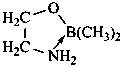
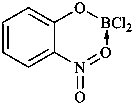
(O—B)-2-nitrophenyl borodichloridate
(O—B)dichloro(2-nitrophenoxy)borane
dichlorido(2-nitro-κO-phenolato-κO)boron
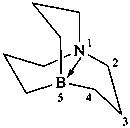
(N—B)-1-aza-5-borabicyclo[3.3.3]undecane (numbering shown)
[3,3′,3′′-nitrilo-κN-tris(propan-1-yl-κC1)]boron
[3,3′,3′′-nitrilo-κN-tris(propan-1-ido-κC1)]boron
Note: If the structure is shown as a charged system, it is named as a zwitterion (see P-74.1.1).
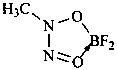
(O—B)-N-[(difluoroboranyl)oxy]-N-nitrosomethanamine
difluorido(N-nitroso-κO-N-oxido-κO-methanamine)boron
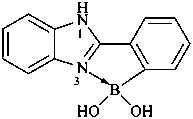
(N3—B)[2-(1H-1,3-benzimidazol-2-yl)phenyl]boronic acid
(N3—B)[2-(1H-benzimidazol-2-yl)phenyl]boronic acid
{not (N3—B)-2-[(dihydroxyboranyl)phenyl]benzimidazole}
[2-(1H-1,3-benzimidazol-2-yl-κN3)benzen-1-ido-κC1]dihydroxidoboron
[2-(1H-benzimidazol-2-yl-κN3)benzen-1-ido-κC1]dihydroxidoboron
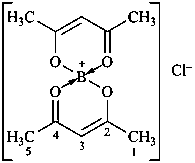
[2(O—B)]bis[(4-oxopent-2-en-2-yl)oxy]boranylium chloride
bis(4-oxo-κO-pent-2-en-2-olato-κO)boron(1+) chloride
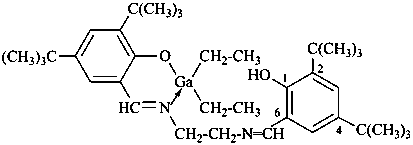
2,4-di-tert-butyl-6-[({2-[({(N—Ga)-3,5-di-tert-butyl-2-[(diethylgallanyl)-oxy]phenyl}methylidene)amino]ethyl}imino)methyl]phenol
[2,4-di-tert-butyl-6-{[(2-{[(3,5-di-tert-butyl-2-hydroxyphenyl)methylidene]amino}ethyl)imino-κN]methyl}phenolato-κO]diethanidogallium
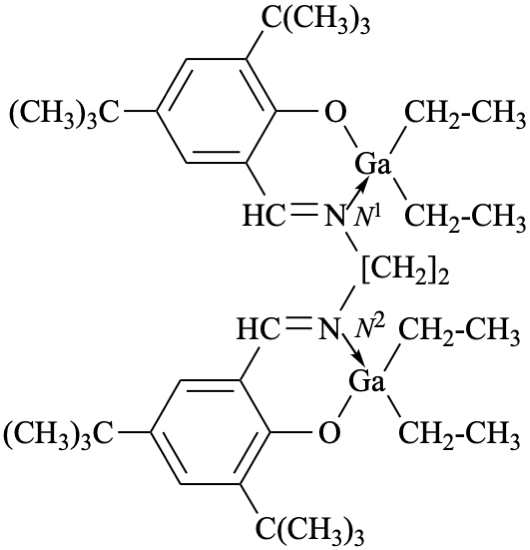
N1,N2-bis({3,5-di-tert-butyl-2-[(diethylgallanyl)oxy]phenyl}methylidene)-2(N—Ga)-ethane-1,2-diamine
(μ-{2,2′-[ethane-1,2-diylbis(azanylylidene-1κN1:2κN2-methanylylidene)bis(4,6-di-tert-butylphenolato-1κO:2κO′)]})bis[di(ethanido-1κ2C1,2κ2C1)gallium]
P-68.2.0 Introduction
The nomenclature of carbon compounds as the basis for organic compounds, has been described in previous chapters. It is not exemplified in this Section, unless comparisons with other compounds are needed.
With the exception of silicic acid, a retained name for Si(OH)4 (formerly orthosilic acid), all silicon compounds and a large number of germanium, tin, and lead compounds are named in accordance with the principles, rules and conventions of substitutive nomenclature.
Germanium, tin, and lead compounds that do not contain carbon or that cannot be named by the principles of substitutive nomenclature as defined in these recommendations for carbon compounds are named by the principles of coordination nomenclature (see ref. 12).
Suffixes are now used in accordance with the seniority of classes (see P-41) for germanium, tin, and lead compounds, which is a change from the traditional system where prefixes only were used for compounds of these elements.
P-68.2.1 Silicon, germanium, tin, and lead parent hydridesP-68.2.1 Silicon, germanium, tin, and lead parent hydrides
P-68.2.2 Substituent groups derived from parent hydrides
P-68.2.3 Modification of the degree of hydrogenation
P-68.2.4 Silicic acid as a functional parent compound
P-68.2.5 Substitutive nomenclature: suffix mode
P-68.2.6 Substitutive nomenclature: prefix mode
P-68.2.1.1 Mononuclear and acyclic parent hydridesP-68.2.1.1 Mononuclear and acyclic parent hydrides
P-68.2.1.2 Cyclic parent hydrides
Names of acyclic parent hydrides are formed in accordance with the general rules described in Section P-21. They are preselected names.
Examples:
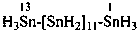
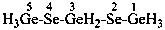
trigermaselenane (preselected name)
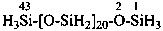
docosasiloxane (preselected name)
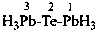
diplumbatellurane (preselected name)
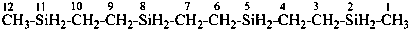
2,5,8,11-tetrasiladodecane (PIN)
ethylenebis[2-(methylsilyl)ethylsilane]
Names and preferred IUPAC names of cyclic parent hydrides are formed in accordance with the rules described in Sections P-22 through P-28. Those with no carbon atoms are preselected.
Examples:
1,3,2-dithiagermolane (PIN)
1,3,5,7,2,4,6,8-tetroxatetragermocane (preselected Hantzsch-Widman name)
cyclotetragermoxane
2,4,6,8,9,10-hexathia-1,3,5,7-tetrasilaadamantane (preselected name)
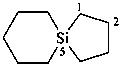
5-silaspiro[4.5]decane (PIN)
Names of substituent groups –XH3, =XH2, and ≡XH derived from mononuclear parent hydrides where X = Si, Ge, Sn, and Pb, but not B, are formed by the method (1) described in P-29.2; all other substituent groups are named by using the general method (2) described in P-29.2.
Examples:
–GeH3
germyl (preselected prefix)
–SnH3
stannyl (preselected prefix)
–PbH3
plumbyl (preselected prefix)
–SiH2–
silanediyl (preselected prefix)
(not silylene)
–GeH2–
germanediyl (preselected prefix)
(not germylene)
–SnH2–
stannanediyl (preselected prefix)
(not stannylene)
–PbH2–
plumbanediyl (preselected prefix)
(not plumbylene)
=SiH2
silylidene (preselected prefix)
(not silylene)
=PbH2
plumbylidene (preselected prefix)
(not plumbylene)
≡GeH
germylidyne (preselected prefix)
≡SnH
stannylidyne (preselected prefix)
(not stannylene)
≡SiH
silylidyne (preselected prefix)
–SiH=
silanylylidene (preselected prefix)
–SnH<
stannanetriyl (preselected prefix)
=Ge=
germanediylidene (preselected prefix)
>Pb<
plumbanetetrayl (preselected prefix)
H3Si-SiH2–
disilanyl (preselected prefix)
(not disilyl)
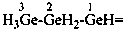
trigerman-1-ylidene (preselected prefix)
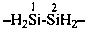
disilane-1,2-diyl (preselected prefix)
disilane-1,1-diyl (preselected prefix)
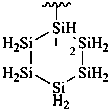
hexasilinanyl (preselected prefix)
cyclohexasilanyl
2-benzosilin-2-yl (preferred prefix)
Double bonds are denoted by the ‘ene’ ending, as described in Section P-31.1, and saturation of double bonds of mancude structures by ‘hydro’ prefixes as indicated in P-31.2.
In these recommendations, the prefixes ‘hydro’ and ‘dehydro’ are detachable, but are not included in the category of alphabetized detachable prefixes (see P-14.4; see also P-15.1.5.2, P-31.2, P-58.2). This is a change from recommendations in earlier editions (ref. 1, 2).
Examples:
1,2,3,4-tetrahydrogermine (PIN)
The nomenclature of silicic acid (formerly orthosilicic acid) has been discussed in Section P-67.1.2. Silicic acid is modified only by prefixes to denote functional replacement by chalcogen atoms. Functional replacement by other atoms or groups is not recommended. Names of salts, esters, and anhydrides are derived from the retained name silicic acid. Amides of silicic acid are classified as amines. Hydrazides of silicic acid are considered as derivatives of hydrazine.
Names of substituent groups derived from silicic acid are formed on the basis of the parent hydride ‘silane’ (see P-67.1.4.2).
Examples:
(HS)(HO)2Si–
dihydroxy(sulfanyl)silyl (preselected name)
Si(NH2)4
silanetetramine (preselected name)
(not silicic tetraamide)
Si(NH-NH2)4
1,1′,1′′,1′′′-silanetetrayltetrahydrazine (preselected name)
(not silicic tetrahydrazide)
Traditionally, silicon compounds were denoted by suffixes or prefixes; germanium, tin, and lead compounds were denoted only by prefixes. Full systematization is recommended to use suffixes, when available, to generate preferred names, in accordance with the seniority order of suffixes, and the seniority of suffixes over prefixes.
Examples:
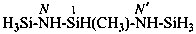
1-methyl-N,N′-disilylsilanediamine (PIN)
(not 3-methyltrisilazane)
(CH3)2Si(OH)2
dimethylsilanediol (PIN)
CH3-NH-Si(OH)3
(methylamino)silanetriol (PIN)
(CH3)3Si-COOH
trimethylsilanecarboxylic acid (PIN)
CH3-GeH2-SH
methylgermanethiol (PIN)
methyl(sulfanyl)germane
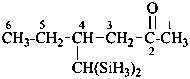
4-(disilylmethyl)hexan-2-one (PIN)
4-[bis(silanyl)methyl]hexan-2-one
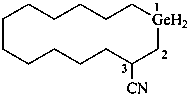
1-germacyclotetradecane-3-carbonitrile (PIN)
3-cyano-1-germacyclotetradecane
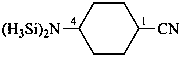
4-(disilylamino)cyclohexane-1-carbonitrile (PIN)
4-[bis(silanyl)amino]cyclohexane-1-carbonitrile
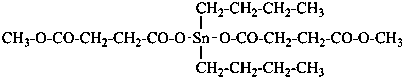
dimethyl dibutylstannanediyl dibutanedioate (PIN; see P-65.6.3.3.4)
dibutylbis[(4-methoxy-4-oxobutanoyl)oxy]stannane
[(CH3)3Si]2CH-SnH(OH)-CH[Si(CH3)3]2
bis[bis(trimethylsilyl)methyl]stannanol (PIN)
[(hydroxystannanediyl)dimethanetriyl]tetrakis(trimethylsilane)
Prefixes are used as recommended for substitutive nomenclature in two ways.
P-68.2.6.1 Subsititued parent hydridesWhen required, parent structures and prefixes are chosen in accordance with the seniority of classes (see P-41).
P-68.2.6.2 Seniority of the Group 14 elements
P-68.2.6.1 Substituted parent hydrides
Examples:
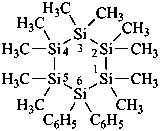
1,1,2,2,3,3,4,4,5,5-decamethyl-6,6-diphenylhexasilinane (PIN; Hantzsch-Widman name)
1,1,2,2,3,3,4,4,5,5-decamethyl-6,6-diphenylcyclohexasilane
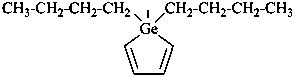
1,1-dibutyl-1H-germole (PIN)
(note the indicated hydrogen atom)
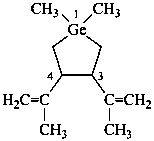
1,1-dimethyl-3,4-di(prop-1-en-2-yl)germolane (PIN)
3,4-diisopropenyl-1,1-dimethylgermolane
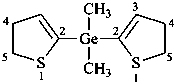
bis(4,5-dihydrothiophen-2-yl)di(methyl)germane (PIN)
(Ge is senior to S)
In the presence of groups of higher seniority, parent structures and prefixes are chosen according to the order of seniority (see P-41 and P-44) and the rules for selection of a preferred name (see P-45).
Examples:
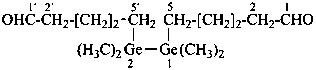
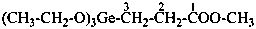
methyl 3-(triethoxygermyl)propanoate (PIN)
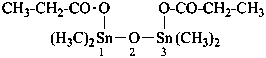
1,1,3,3-tetramethyldistannoxane-1,3-diyl dipropanoate (PIN)
H3Pb-CH2-PbH2-CH2-PbH3
[plumbanediylbis(methylene)]bis(plumbane) (PIN)
bis(plumbylmethyl)plumbane
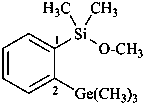
methoxydi(methyl)[2-(trimethylgermyl)phenyl]silane (PIN)
(Si is senior to Ge)
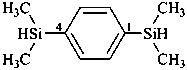
(1,4-phenylene)bis(dimethylsilane) (PIN)
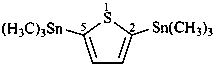
(thiophene-2,5-diyl)bis(trimethylstannane) (PIN)
(Sn preferred to S, see P-41)
[(CH3)3Si]2CH-SnH(OH)-CH[Si(CH3)3]2
bis[bis(trimethylsilyl)methyl]stannanol (PIN)
[(hydroxystannanediyl)dimethanetriyl]tetrakis(trimethylsilane)
[Decided by P-41 table 4.1 class 17, not class 26 or class 28]
For the purposes of nomenclature, compounds of the Group 15 elements are divided into three categories:
(1) Nitrogen compounds are named as amines and imines (see P-62), amides, hydrazides, imides, amidines, amidrazones, hydrazidines, nitriles and cyanides (see P-66), or substitutively on the basis of parent hydrides, such as hydrazine, triazane, etc., or as derivatives of functional parent compounds, such as hydroxylamine (see P-68.3.1.1). Because of similarities, azonic HN(O)(OH)2 and azinic acids H2N(O)(OH), are discussed in Section P-67 along with the P, As, and Sb oxoacids.
(2) Phosphorus, arsenic, and antimony compounds are discussed together in P-68.3.2 because of the importance of functional class nomenclature based on their acids used as functional parents. Other compounds are named substitutively on the basis of parent hydrides.
(3) Bismuth compounds that contain carbon and that can be named by the principles of substitutive nomenclature as described in these recommendations are named by substitution of parent hydrides, such as bismuthane, BiH3 (see P-68.3.3). Bismuth compounds that do not contain carbon or that cannot be named by the principles of substitutive nomenclature as defined in these recommendations for carbon compounds are named by the principles of coordination nomenclature (see ref. 12).
P-68.3.1 Nitrogen compounds
P-68.3.1.0 IntroductionP-68.3.1.0 Introduction
P-68.3.1.1 Hydroxylamines, oximes, and nitrolic acids and nitrosolic acids
P-68.3.1.2 Hydrazine and related compounds: hydrazones, azines, semicarbazides, semicarbazones, and carbonohydrazides
P-68.3.1.3 Diazene and related compounds
P-68.3.1.4 Polyazanes
Many acyclic nitrogen compounds have retained names or functional class names. These names are retained for use in general nomenclature, but for most acyclic nitrogen compounds preferred IUPAC names are formed systematically. Hydroxylamine is a preselected name and urea, guanidine, and formazan are retained as preferred IUPAC names. Other retained names have been inserted into systematic substitutive nomenclature; oxime is retained only as a functional class name.
P-68.3.1.1 Hydroxylamines, oximes, and nitrolic and nitrosolic acids
Compounds containing a nitrogen atom which belong to classes identified as hydroxylamines, oximes, and nitrolic and nitrosolic acids are illustrated here. Nitro and nitroso compounds, isocyanates, and isonitriles were discussed in Section P-61.
The class name ‘hydroxylamine’, a preselected name, is also used as a functional parent compound. The class name ‘oxime’ is used as a functional class modifier. Nitrolic and nitrosolic acids are named as oximes of pseudoketones.
P-68.3.1.1.1 HydroxylaminesP-68.3.1.1.1 Hydroxylamines
P-68.3.1.1.2 Oximes
P-68.3.1.1.3 Nitrolic and nitrosolic acids
The retained name ‘hydroxylamine’ is a preselected name and designates the structure H2N-OH. It is a functional parent compound allowing full substitution even, as an exception, on the oxygen atom. Substitution on the nitrogen or the oxygen atom of hydroxylamine may create a function senior to hydroxylamine, justifying a systematic name based on a higher class denoting the new function.
P-68.3.1.1.1.1 Substituted hydroxylamines of the type R-NH-OH or RR′N-OH are named as N-derivatives of the senior amine.
Examples:
(CH3)2N-OH
N-hydroxy-N-methylmethanamine (PIN)
N,N-dimethylhydroxylamine
H3Si-NH-OH
N-hydroxysilanamine (preselected name)
N-silylhydroxylamine
H2B-NH-OH
N-hydroxyboranamine (preselected name)
N-boranylhydroxylamine
Examples:
CH3-SO2-NH-OH
N-hydroxymethanesulfonamide (PIN)
CH3-CH2-CO-NH-OH
N-hydroxypropanamide (PIN)
propanohydroxamic acid
propionohydroxamic acid
Substitution of hydroxylamine on the oxygen atom by hydrocarbyl or acyl groups is expressed as O-substitution. Names such as ‘alkyloxyamines’ are not recommended and the class ‘peroxyamide’ has never been recognized.
Examples:
H2N-O-C6H5
O-phenylhydroxylamine (PIN)
(not phenoxyamine)
H2N-O-CH2-CH2-O-NH2
O,O′-(ethane-1,2-diyl)bis(hydroxylamine) (PIN)
H2N-O-CO-C6H5
aminooxy(phenyl)methanone (PIN)
O-benzoylhydroxylamine
(not azanyl benzoate)
H2N-O-SO-CH3
O-(methanesulfinyl)hydroxylamine (PIN)
(not azanyl methanesulfinate)
Example:
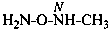
Substitution of hydroxylamine on both nitrogen and oxygen atoms leads to O-substituted amines.
Examples:
C6H5-NH-O-CH2-CH3
N-ethoxyaniline (PIN)
O-ethyl-N-phenylhydroxylamine
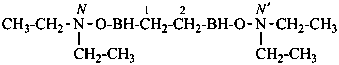
N,N′-[ethane-1,2-diylbis(boranediyloxy)]bis(N-ethylethanamine) (PIN)
O,O′-[ethane-1,2-diylbis(boranediyl)]bis(N,N-diethylhydroxylamine)
Note: Skeletal (‘a’) replacement nomenclature cannot be used for this compound because the amine characteristic group would not be recognized and an ‘a’ chain cannot terminate on an oxygen atom.
Hydroxylamine is a functional parent to which, exceptionally, suffixes expressing characteristic groups, such as acids, amides, etc., can be attached to the oxygen atom. In names, the locant ‘O’ is placed in front of the suffix.
Attachment of characteristic groups to the nitrogen atom generally leads to compounds of higher function.
Examples:
H2N-O-COOH
hydroxylamine-O-carboxylic acid (PIN)
(not azanyl hydrogen carbonate)
H2N-O-CO-NH2
hydroxylamine-O-carboxamide (PIN)
(not azanyl carbamate)
In the presence of a characteristic group having priority for citation as suffix or in the presence of a senior parent hydride, appropriate compound or complex prefixes are used:
>N-OH
hydroxyazanediyl (preselected prefix)
–O-NH2
aminooxy (preselected prefix)
(note that there is no elision of the final letter ‘o’ of amino)
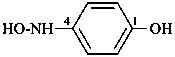
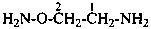
2-(aminooxy)ethan-1-amine (PIN)
H2N-NH-CH2-O-NH2
[(aminooxy)methyl]hydrazine (PIN)
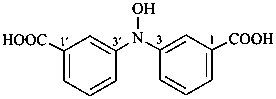
3,3′-(hydroxyazanediyl)dibenzoic acid (PIN)
Chalcogen analogues of hydroxylamine are denoted by the appropriate functional replacement prefix ‘thio’, ‘seleno’, or ‘telluro’. Parentheses are sometimes required to avoid the possibility of ambiguity. Substitution follows the principles given above for naming derivatives of hydroxylamine.
Examples:
CH3-NH-SH
N-sulfanylmethanamine (PIN)
N-methyl(thiohydroxylamine)
CH3-CO-NH-SH
N-sulfanylacetamide (PIN)
H2N-S-CH3
S-methyl(thiohydroxylamine) (PIN)
Compounds having the general structure R-CH=N-OH or RR′C=N-OH have the class name ‘oxime’ and have been further classified as ‘aldoximes’ and ‘ketoximes’, respectively. For general nomenclature they are named according to the principles of functional class nomenclature by placing the class name ‘oxime’ as a separate word after the name of the aldehyde or ketone. Preferred IUPAC names are formed substitutively as N-hydroxy derivatives of imines. Compounds containing the group =N-OR are named substitutively as alkoxy substituted imines. In the presence of a characteristic group having priority for citation as a suffix, in substitutive nomenclature oximes are designated by the prefix ‘hydroxyimino’.
In these recommendations preferred IUPAC names for oximes are generated substitutively as N-hydroxy derivatives of imines rather than by functional class nomenclature as in previous recommendations
Examples:
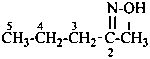
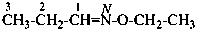
N-ethoxypropan-1-imine (PIN)
propanal O-ethyloxime
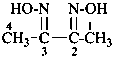
N2,N3-dihydroxybutane-2,3-diimine (PIN)
(butane-2,3-diylidene)bis(hydroxylamine)
butane-2,3-dione dioxime
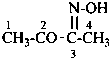
3-(hydroxyimino)butan-2-one (PIN)
butane-2,3-dione oxime
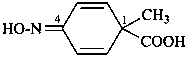
4-(hydroxyimino)-1-methylcyclohexa-2,5-diene-1-carboxylic acid (PIN)
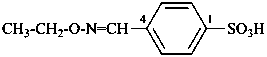
4-[(ethoxyimino)methyl]benzene-1-sulfonic acid (PIN)
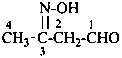
3-(hydroxyimino)butanal (PIN)
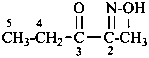
2-(hydroxyimino)pentan-3-one (PIN)
pentane-2,3-dione 2-oxime
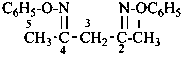
N2,N4-diphenoxypentane-2,4-diimine (PIN)
pentane-2,4-dione bis(O-phenyloxime)
Compounds having the general structures R-C(=NOH)-NO2 and R-C(=NOH)-NO are called generically ‘nitrolic acids’ and ‘nitrosolic acids’, respectively. They are named substitutively for general nomenclature as oximes of pseudoketones. Preferred IUPAC names are formed as described above for ‘oximes’ (see P-68.3.1.1.2). Traditionally, they were named by functional class nomenclature as oximes of aldehydes substituted in position 1 by a nitro or nitroso group.
Examples:
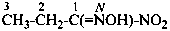
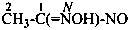
N-hydroxy-1-nitrosoethan-1-imine (PIN)
N-(1-nitrosoethylidene)hydroxylamine (PIN)
1-nitrosoacetaldehyde oxime)
1-nitrosoethan-1-one oxime
P-68.3.1.2.1 Hydrazine and derivatives
Hydrazine is a retained name describing the structure H2N-NH2; it is a preselected name and is preferred to the systematic heterane name ‘diazane’.
Substituent groups derived from hydrazine are named systematically:
Preselected prefixes derived from the preselected parent hydride hydrazine are now formed systematically from hydrazine. For H2N-NH– ‘hydrazinyl’; for H2N-N= ‘hydrazinylidene’; for =N-N= ‘hydrazinediylidene’; and for –NH-NH– ‘hydrazine-1,2-diyl’. The traditional names ‘hydrazino’, ‘hydrazono’, ‘azino’, and ‘hydrazo’, respectively, are no longer recommended, even for general nomenclature.
H2N-N=
hydrazinylidene (preselected prefix)
diazanylidene
(not hydrazono)
=N-N=
hydrazinediylidene (preselected prefix)
diazanediylidene
(not azino)
–NH-NH–
hydrazine-1,2-diyl (preselected prefix)
diazane-1,2-diyl
(not hydrazo)
Examples:
C6H5-NH-NH2
phenylhydrazine (PIN)
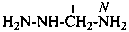
1-hydrazinylmethanamine (PIN)
(see also P-62.2.1.2)
H2N-NH-COOH
hydrazinecarboxylic acid (PIN)
(not carbazic acid)
F2N-NF2
tetrafluorohydrazine
(from preselected name hydrazine)
H2N-NH-CH2-CN
hydrazinylacetonitrile (PIN)
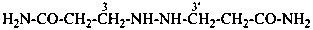
3,3′-(hydrazine-1,2-diyl)dipropanamide (PIN)
(not 3,3′-hydrazodipropanamide)
Compounds having the general structure RCH=N-NH2 or RR′C=N-NH2 are called ‘hydrazones’ and are named in two ways:
(1) substitutively as derivatives of the parent hydride ‘hydrazine’, H2N-NH2;Method (1) generates preferred IUPAC names.(2) by functional class nomenclature using the class name ‘hydrazone’.
In these recommendations preferred IUPAC names for hydrazones are generated substitutively as ‘ylidene’ derivatives of hydrazine rather than by functional class nomenclature as in previous recommendations.
Examples:
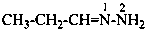
(CH3)2N-N=C(CH3)2
1,1-dimethyl-2-(propan-2-ylidene)hydrazine (PIN)
1,1-dimethyl-2-(1-methylethylidene)hydrazine
acetone dimethylhydrazone
C6H5-NH-N=CH-CH=N-NH-C6H5
1,1′-ethanediylidenebis(2-phenylhydrazine) (PIN)
ethane-1,2-dione bis(phenylhydrazone)
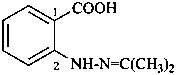
2-[(propan-2-ylidene)hydrazinyl]benzoic acid (PIN)
2-[(1-methylethylidene)hydrazinyl]benzoic acid
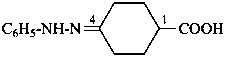
4-(phenylhydrazinylidene)cyclohexane-1-carboxylic acid (PIN)
P-68.3.1.2.3.1 Compounds having the general structure R-CH=N-N=CH-R or RR′C=N-N=RR′ are called ‘azines’ and are named in two ways:
(1) substitutively, as derivatives of hydrazine;Method (1) leads to preferred IUPAC names.(2) by functional class nomenclature using the class name ‘azine’.
In these recommendations preferred IUPAC names for azines are generated substitutively as ‘ylidene’ derivatives of hydrazine rather than by functional class nomenclature as in previous recommendations.
Example:
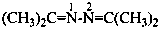
(1) as unsymmetrical derivatives of hydrazine;Method (1) generates preferred IUPAC names.(2) as ‘ylidenehydrazones’ of the preferred ketone or aldehyde.
Example:
Examples:
4,4′-hydrazinediylidenedi(cyclohexane-1-carboxylic acid) (PIN)
Semicarbazide is the amide of ‘hydrazinecarboxylic acid’. As acids and amides expressed by suffixes are senior to acids or amides modified by functional replacement, these names are senior to ‘carbonohydrazidic acid’ and ‘carbonohydrazidic amide’.
Semicarbazide is the traditional name for the compound H2N-NH-CO-NH2, systematically named ‘hydrazinecarboxamide’. The systematic name is the preferred IUPAC name. The usual numbering for amides is recommended for the systematic name; special numbering is characteristic for the name semicarbazide.
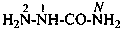
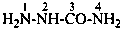
semicarbazide
Numerical locants are no longer used for the parent name semicarbazide in preferred IUPAC names.
Examples:
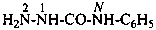
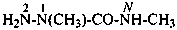
N,1-dimethylhydrazine-1-carboxamide (PIN)
2,4-dimethylsemicarbazide
Example:
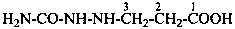
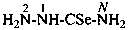
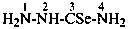
selenosemicarbazide
Compounds with the structure R-CH=N-NH-CO-NR′R′′ or RR′C=N-NH-CO-NR′′R′′′ are generically called ‘semicarbazones’. They are named in two ways:
(1) substitutively by using the functional parent ‘hydrazinecarboxamide’;Method (1) yields preferred IUPAC names.(2) by the class modifier ‘semicarbazone’ placed after the name of the corresponding aldehyde or ketone.
Numerical locants are no longer used for the functional class name semicarbazone in preferred IUPAC names.
Example:
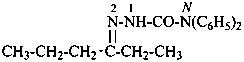
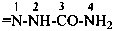
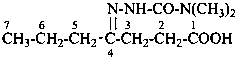
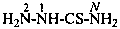
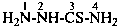
thiosemicarbazide
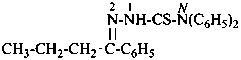
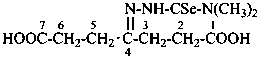
4-[(dimethylcarbamoselenoyl)hydrazinylidene]heptanedioic acid (PIN)
4-[4,4-dimethyl(selenosemicarbazono)]heptanedioic acid
The preferred IUPAC name for the compound H2N-NH-CO-NH-NH2 is ‘hydrazinecarbohydrazide’. The name ‘carbonic dihydrazide’ is also recommended, but only for general nomenclature; the names ‘carbonohydrazide’, ‘carbohydrazide’, and ‘carbazide’ are not recommended. Systematic numbering is applied to the systematic name; a special numbering is assigned to carbonic dihydrazide, as follows:
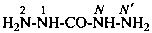
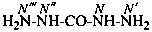
carbonic dihydrazide
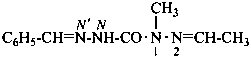
Example:
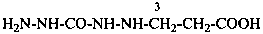
P-68.3.1.3.1 Substitution of diazeneP-68.3.1.3.1 Substitution of diazene
P-68.3.1.3.2 Azo compounds, R-N=N-R′
P-68.3.1.3.3 Azoxy compounds, R-N=N(O)-R′
P-68.3.1.3.4 Diazenecarbohydrazide, HN=N-CO-NH-NH2
P-68.3.1.3.5 Formazan, H2N-N=CH-N=NH
P-68.3.1.3.6 Carbodiazone [bis(diazenyl)methanone], HN=N-CO-N=NH
P-68.3.1.3.7 Isodiazene, R2N+=N–
Diazene is a modified parent hydride derived from the parent hydride diazane (see P-68.3.1.2.1) The prefix ‘diazenyl’ is a preselected prefix (see P-32.1.1).
Examples:
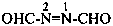
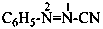
phenyldiazenecarbonitrile (PIN)
CH3-N=N-CH2-COOH
(methyldiazenyl)acetic acid (PIN)
HN=N-CH2-CH2-COOH
3-diazenylpropanoic acid (PIN)
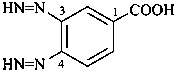
3,4-bis(diazenyl)benzoic acid (PIN)
Note: Rules for naming azo compounds using the azo prefix were quite complex in the 1979 recommendations (ref. 1). Two sets of rules were recommended, the so-called ‘old method’ (Rule C-911) and the method used in CAS index nomenclature (Rule C-912) before 1972. The ‘old method’ (Rule C-911, ref. 1) must be totally discarded because some examples do not follow the basic rules developed in the 1979 organic nomenclature rules (ref. 1), especially with respect to the treatment of suffixes. In 1993, a new approach, introduced by CAS in 1972, was adopted based on the use of the parent hydride name ‘diazene’ [Method (1), below]. It brought simplicity and rationalization to the field and is the method chosen to generate preferred IUPAC names in these recommendations. However, names based on the method given as Rule C-912 in the 1979 recommendations are given here as an acceptable alternative for general nomenclature.
Compounds with the general structure R-N=N-R′, where R and R′ may be alike or different, are known generically as ‘azo compounds’. They are named in two ways:
(1) substitutively using the parent ‘diazene’, HN=NH;Method (1) leads to preferred IUPAC names.(2) by using the prefix ‘azo’ in the traditional manner (P-68.3.1.3.2.1).
Azo compounds are divided into monoazo compounds, having one –N=N– group, bis(azo) compounds, having two –N=N– groups, and so on.
P-68.3.1.3.2.1 Symmetrical monoazo compounds, R-N=N-R, are named:
(1) by substituting the parent diazene, HN=NH, by the appropriate substituent groups;Method (1) gives preferred IUPAC names.(2) by adding ‘azo’ to the name of the parent hydride, RH; substituents are denoted in the usual way by prefixes, the two RH parents being distinguished by unprimed and primed locants. Attachment of the azo group has priority for lowest available numbers.
Examples:
C6H5-N=N-C6H5
diphenyldiazene (PIN)
azobenzene
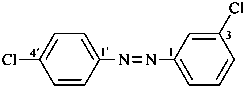
(3-chlorophenyl)(4-chlorophenyl)diazene (PIN)
3,4′-dichloroazobenzene (numbering shown)
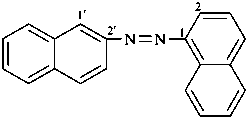
(naphthalen-1-yl)(naphthalen-2-yl)diazene (PIN)
1,2′-azonaphthalene (numbering shown)
(1) substitutively, by prefixing the names of the appropriate substituent groups, in alphabetical order, before the parent hydride name diazene;Method (1) leads to preferred IUPAC names.(2) by inserting ‘azo’ between the names of the parent hydrides RH and R′H; the principal chain or the senior ring or ring system is cited first and is assigned plain locants, the other parent hydride being given primed locants; when locants are required to denote the points of attachment of the parent hydrides, they are placed immediately before or after the prefix ‘azo’, respectively.
Examples:
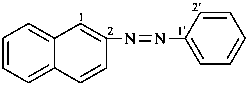
(naphthalen-2-yl)(phenyl)diazene (PIN)
naphthalene-2-azobenzene (numbering shown)
Examples:
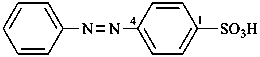
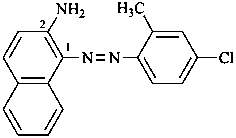
1-[(4-chloro-2-methylphenyl)diazenyl]naphthalen-2-amine (PIN)
[not 2-aminonaphthalene-1-azo(4′-chloro-2′-methylbenzene) (see C-911.2, ref. 1);
the principal characteristic group, the amine, must be denoted by a suffix]
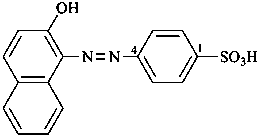
4-[(2-hydroxynaphthalen-1-yl)diazenyl]benzene-1-sulfonic acid (PIN)
4-[(2-hydroxy-1-naphthyl)azo]benzene-1-sulfonic acid
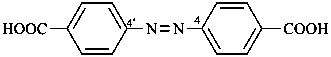
4,4′-diazenediyldibenzoic acid (PIN)
(traditionally 4,4′-azodibenzoic acid)
(1) on the basis of ‘diazene’, as described in P-68.3.1.3.2.1 above, the first cited substituent being chosen on the basis of the principle of alphanumerical order;Method (1) generates preferred IUPAC names.(2) by using the prefix ‘azo’, as described in P-68.3.1.3.2.1;
(3) by using the prefix ‘azo’ and, after choosing the principal parent hydride, substituting the other components as an ‘organylazo’ group.
Example:
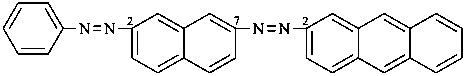
Example:
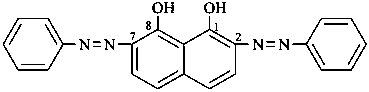
P-68.3.1.3.3.1 N-Oxides of azo compounds having the general structure R-N2(O)-R′ (R = R′ or R ≠ R′) are known generically as ‘azoxy compounds’. Their nomenclature was revised in 1993 (ref. 2) and is used in these recommendations. Azoxy compounds are named in two different ways:
(1) by adding the term ‘oxide’ to the name of the corresponding azo compound, preceded by a locant 1 or 2;Method (1) leads to preferred IUPAC names. Azoxy compounds could also be named by using the λ-convention or as zwitterions.(2) in the traditional way of replacing the prefix ‘azo’ by ‘azoxy’ and using the locants NNO and ONN to indicate the parent hydride associated with the =N(O) group. In the general structure R-N(O)N-R′, the symbol NNO specifies that the oxygen atom is attached to the nitrogen atom next to the R′ group. The symbol ONN specifies that the oxygen atom is attached to the nitrogen atom next to the R group. When the point of attachment is not known, the symbol NON is used.
Examples:
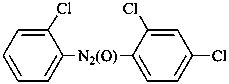
(2-chlorophenyl)(2,4-dichlorophenyl)diazene oxide (PIN)
(the absence of locants indicates that the points of attachment are not known)
2,2′,4-trichloroazoxybenzene
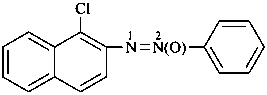
1-(1-chloronaphthalen-2-yl)-2-phenyldiazene 2-oxide (PIN)
1-chloro-2-(phenyl-ONN-azoxy)naphthalene
Example:

Example:
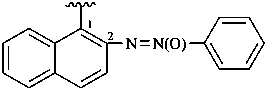
The hydrazide of ‘diazenecarboxylic acid’, HN=N-CO-NH-NH2, is named systematically ‘diazenecarbohydrazide’; it is the preferred IUPAC name. The name ‘carbazone’ is not recommended. Chalcogen analogues are named by using the infixes and the prefixes ‘thio’, ‘seleno’, and ‘telluro’.
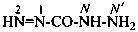
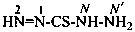
diazenecarbothiohydrazide (PIN)
Examples:
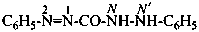
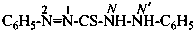
N′,2-diphenyldiazenecarbothiohydrazide (PIN)
The group H2N-NH-CO-N=N– is named ‘(hydrazinecarbonyl)diazenyl’.
Example:
The hydrazone of diazenecarbaldehyde, H2N-N=CH-N=NH, has the retained name, which is also the preferred IUPAC name ‘formazan’; it is numbered in a special manner. It can also be named substitutively as a derivative of the parent hydride ‘hydrazine’; its derivatives are named accordingly.
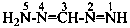
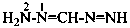
(diazenylmethylidene)hydrazine
Preferred names are constructed systematically as derivatives of formazan when prefixes only are present or when the principal characteristic group is attached directly to the formazan structure. Otherwise, the functionalized formazan parent must be expressed according to the seniority rules applied in constructing names (see P-4).
Examples:
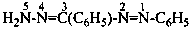
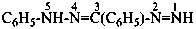
3,5-diphenylformazan (PIN)
[diazenyl(phenyl)methylidene]phenylhydrazine
phenyl(diazenyl)methanone 2-phenylhydrazone
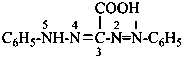
1,5-diphenylformazan-3-carboxylic acid (PIN)
(phenyldiazenyl)(phenylhydrazinylidene)acetic acid
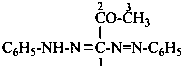
1-(phenyldiazenyl)-1-(phenylhydrazinylidene)propan-2-one (PIN)
3-acetyl-1,5-diphenylformazan
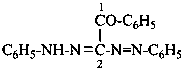
1-phenyl-2-(phenyldiazenyl)-2-(phenylhydrazinylidene)ethan-1-one (PIN)
3-benzoyl-1,5-diphenylformazan
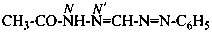
N′-[(phenyldiazenyl)methylidene]acetohydrazide (PIN)
Substituent groups derived from formazan are as follows; retained names lead to preferred IUPAC prefixes.
| Preferred prefix | Systematic prefix | |||
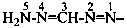 | formazan-1-yl | (hydrazinylidenemethyl)diazenyl | ||
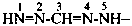 | formazan-5-yl | (diazenylmethylidene)hydrazinyl | ||
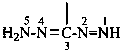 | formazan-3-yl | diazenyl(hydrazinylidene)methyl | ||
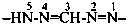 | formazan-1,5-diyl | |||
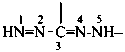 | formazan-3,5-diyl | |||
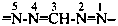 | formazan-1-yl-5-ylidene | |||
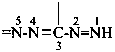 | formazan-3-yl-5-ylidene | |||
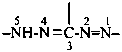 | formazan-1,3,5-triyl |
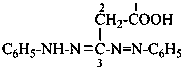
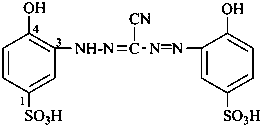
3,3′-(3-cyanoformazan-1,5-diyl)bis(4-hydroxybenzene-1-sulfonic acid) (PIN)
3-(2-{cyano[(2-hydroxy-5-sulfophenyl)diazenyl]methylidene}hydrazinyl)-4-hydroxybenzene-1-sulfonic acid
{not 3-({cyano[(2-hydroxy-5-sulfophenyl)hydrazinylidene]methyl}diazenyl)-4-hydroxybenzene-1-sulfonic acid
(‘cyanohydroxysulfophenyldiazenyl...’ is lower alphabetically than ‘cyanohydroxysulfophenylhydrazinylidene...’}
The compound HN=N-CO-N=NH is named systematically bis(diazenyl)methanone; its hydrocarbyl derivatives are named substitutively. Such names are preferred IUPAC names over those denoted by the retained name ‘carbodiazone’, which can be used in general nomenclature with full substitution according to a special numbering. The name ‘bis(diazenyl)methanone’ is preferred to 1,1′-carbonylbis(diazene) simply because a ketone, expressed by the suffix ‘one’, is senior to the parent structure ‘diazane’ or ‘diazene’ (see P-41).
Chalcogen analogues of bis(diazenyl)methanone are also named systematically as bis(diazenyl)methanethione, -selone, and -tellone, respectively. The prefixes thio, seleno, and telluro are used with the name carbodiazone in general nomenclature.
Examples:
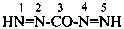
carbodiazone
HN=N-CS-N=NH
bis(diazenyl)methanethione (PIN)
thiocarbodiazone
HN=N-CO-N=N–
(diazenecarbonyl)diazenyl (preferred prefix)
C6H5-N=N-CO-N=N-C6H5
bis(phenyldiazenyl)methanone (PIN)
1,5-diphenylcarbodiazone
Compounds with the general structure R2N-N: ↔ R2N+=N– are called ‘isodiazenes’ generically and are named substitutively on the basis of the parent radical ‘hydrazinylidene’, H2N-N:. This method leads to preferred IUPAC names rather than the names based on the parent hydride name ‘isodiazene’.
Example:
P-68.3.1.4.1 Acyclic polyazanes are saturated chains of nitrogen atoms; hydrazine is the retained name and the preselected name for diazane, H2N-NH2. Names are formed by prefixing the mononuclear parent hydride name ‘azane’ with an appropriate numerical prefix and numbered in the same way as hydrocarbons. The parent hydrides are preselected names (see P-12.2). The final ‘a’ of a numerical prefix is not elided before ‘azane’.
Examples:
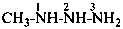
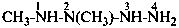
1,2-dimethyltetraazane (PIN)
The formation of systematic prefix names, i.e., triazan-1-yl, etc., as described in P-29, must be considered a change even though the names triazano, tetrazano, etc., previously used as names for bridges in the naming of bridge fused ring systems, are no longer used as such and have been replaced by the names ‘epitriazano’, etc. (see P-25.4.2.1.4).
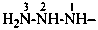

triaz-2-en-1-yl (preselected prefix)
(not triaz-2-eno)
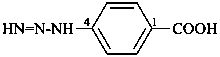
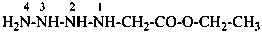
ethyl (tetraazan-1-yl)acetate (PIN)
[not ethyl tetrazanoacetate)
Compounds having the structure R-N=N-NR2 are known as ‘diazoamino compounds’ when the same substituent group is located at each end of the chain. They are named substitutively, on the basis of the parent hydride name ‘triazene’ if there is present no characteristic group to be cited as a suffix. The prefix ‘diazoamino’ for the group –N=N-NH– is no longer recommended.
Examples:
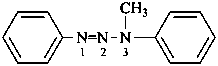
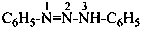
1,3-diphenyltriaz-1-ene (PIN)
(formerly diazoaminobenzene)
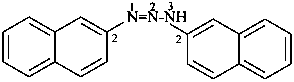
1,3-di(naphthalen-2-yl)triaz-1-ene (PIN)
(formerly 2,2′-diazoaminonaphthalene)
P-68.3.2.1 General methodology
Preferred names of acyclic phosphorus, arsenic, and antimony compounds are functional class names (see P-67) derived from mononuclear and polynuclear acids, such as phosphoric acid, H3PO4, arsonous acid, HAs(OH)2, stibinic acid, H2Sb(O)(OH), and diphosphonic acid, HO-HP(O)-O-P(O)H-OH, rather than substitutive names based on parent hydrides.
Other preferred names of acyclic and cyclic compounds are substitutive names, in accordance with the seniority of classes (see P-41).
This subsection includes the description of functional class nomenclature and substitutive nomenclature.
P-68.3.2.2 Parent hydridesP-68.3.2.2 Parent hydrides
P-68.3.2.3 Substitutive nomenclature
Parent hydrides are formed by the methods described in Chapter P-2. They are mononuclear, acyclic polynuclear, and cyclic ring systems and are preselected names (see P-12.2) The λ-convention is used to denote pentavalent phosphorus and arsenic atoms. Retained names for use only in general nomenclature are phosphine, phosphorane, arsine, arsorane, stibine, and stiborane.
Preferred names are selected as indicated in Chapter P-2.
Examples:
AsH5
λ5-arsane (preselected name)
arsorane
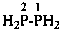
diphosphane (preselected name)
diphosphine
pentaarsane (preselected name)
pentaphospholane (preselected name)
cyclopentaphosphane
1,3,5,2,4,6-triazatriphosphinine (preselected name)
(not cyclotriphosphazene, which has only one double bond)
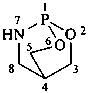
2,6-dioxa-7-aza-1-phosphabicyclo[2.2.2]octane (PIN)
arsinoline (PIN)
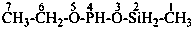
ethyl methylsilyl phosphonite (PIN)
3,5-dioxa-4-phospha-2-silaheptane
1,1′-bistibinane (PIN)
Compounds not named in accordance with the previous section, P-68.3.2.2 are named substitutively on the basis of acyclic and cyclic parent hydrides, using suffixes and prefixes to designate characteristic groups.
P-68.3.2.3.1 Substitutive nomenclature, suffix mode
Suffixes are used to denote characteristic groups present as principal groups, with the exception of acids having retained names and their derivatives, as described in P-67. This method produces preferred IUPAC names over those formed by functional class nomenclature, where =O, =S, =Se, =Te, =NH are denoted by the class names oxide, sulfide, selenide, telluride, and imide added to the name of the parent hydride.
Examples:
H2P-CO-NH2
phosphanecarboxamide (PIN)
C6H5-P=O
phenylphosphanone (PIN)
[not oxo(phenyl)phosphane]
(C6H5)3P=O
triphenyl-λ5-phosphanone (PIN)
triphenylphosphane oxide
(not oxotriphenyl-λ5-phosphane)
HP=N-CH3
N-methylphosphanimine (PIN)
[not (methylimino)phosphane]
(CH3)3As=Te
trimethyl-λ5-arsanetellone (PIN)
trimethylarsane telluride
C6H5-As=S
phenylarsanethione (PIN)
[not phenyl(sulfanylidene)arsane]
(CH3)3As=NH
As,As,As-trimethyl-λ5-arsanimine (PIN)
trimethylarsane imide
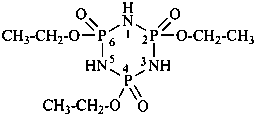
2,4,6-triethoxy-1,3,5,2λ5,4λ5,6λ5-triazatriphosphinane-2,4,6-trione (PIN)
The seniority of classes must be applied in the following order: classes expressed by suffixes, then classes in order of the heteroatom class, i.e., N > P > As > Sb > Bi > Si > Ge > Sn > Pb > B > Al > Ga > In > Tl > O > S > Se > and Te.
P-68.3.2.3.2.1 Substitution of phosphanes, arsanes, and stibanes by organyl groups
Alkyl, aryl, etc. groups and groups derived from parent hydrides containing O, S, Se, and Te atoms are always denoted by prefixes. Halides and pseudohalides are not expressed by prefixes when attached directly to a P, As, or Sb atom, because functional replacement of parent acids having retained names is senior for naming them as acid halides or pseudohalides (see P-67.1.2.5.1).
Examples:
CH3-CH2-AsH2
ethylarsane (PIN)
ethylarsine
P(OCH3)5
pentamethoxy-λ5-phosphane (PIN)
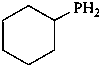
cyclohexylphosphane (PIN)
cyclohexylphosphine
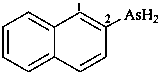
(naphthalen-2-yl)arsane (PIN)
(naphthalen-2-yl)arsine
2-naphthylarsane
ClCH2-CH2-AsH-CHCl-CH3
(1-chloroethyl)(2-chloroethyl)arsane (PIN)
(1-chloroethyl)(2-chloroethyl)arsine
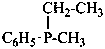
ethyl(methyl)(phenyl)phosphane (PIN)
ethyl(methyl)(phenyl)phosphine
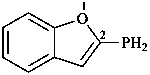
(1-benzofuran-2-yl)phosphane (PIN)
(1-benzofuran-2-yl)phosphine
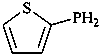
(thiophen-2-yl)phosphane (PIN)
(thiophen-2-yl)phosphine
(CH3)2P-CH2-CH2-P(CH3)2
(ethane-1,2-diyl)bis(dimethylphosphane) (PIN)
(ethane-1,2-diyl)bis(dimethylphosphine)
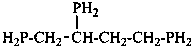
(butane-1,2,4-triyl)tris(phosphane) (PIN)
(butane-1,2,4-triyl)tris(phosphine)
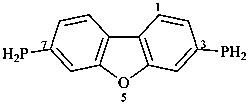
(dibenzo[b,d]furan-3,7-diyl)bis(phosphane) (PIN)
(dibenzofuran-3,7-diyl)bis(phosphine)
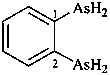
(1,2-phenylene)bis(arsane) (PIN)
(1,2-phenylene)bis(arsine)
Substituent groups are formed by the general method described in Section P-29, i.e., by adding the suffixes ‘yl’, ‘ylidene’ and ‘ylidyne’ to the name of the parent hydride with elision of the final letter ‘e’ of the parent hydride name. When required, the order of classes is applied, as indicated in Section P-41. The traditional names phosphino, arsino, and stibino may be used in general nomenclature.
–As=
arsanylylidene (preselected prefix)
–HP-PH–
diphosphane-1,2-diyl (preselected prefix)
–As<
arsanetriyl (preselected prefix)
–AsH2
arsanyl (preselected prefix)
arsino
–SbH2
stibanyl (preselected prefix)
stibino
–SbH-SbH2
distibanyl (preselected prefix)
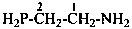
H2As-CH2-P(C6H5)2
(arsanylmethyl)di(phenyl)phosphane (PIN)
(arsinomethyl)di(phenyl)phosphine
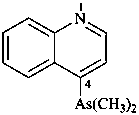
4-(dimethylarsanyl)quinoline (PIN)
4-(dimethylarsino)quinoline
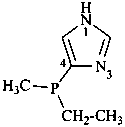
4-[ethyl(methyl)phosphanyl]-1H-imidazole (PIN)
4-[ethyl(methyl)phosphino]-1H-imidazole
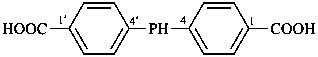
4,4′-phosphanediyldibenzoic acid (PIN)
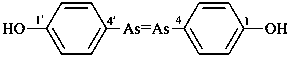
4,4′-diarsenediyldiphenol (PIN)
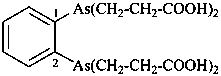
3,3′,3′′,3′′′-[1,2-phenylenebis(arsanetriyl)]tetrapropanoic acid (PIN)
(HO)2As(O)-CH2-COOH
arsonoacetic acid (PIN)
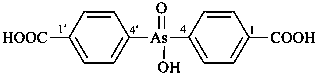
4,4′-(hydroxyarsoryl)dibenzoic acid (PIN)
(not 4,4′-arsinicodibenzoic acid)
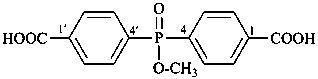
4,4′-(methoxyphosphoryl)dibenzoic acid (PIN)
Cl2P(O)-CH2-CO-Cl
phosphorodichloridoylacetyl chloride (PIN)
(dichlorophosphoryl)acetyl chloride
(CH3O)2P(=NH)-CH2-CO-O-CH3
methyl (P,P-dimethoxyphosphinimidoyl)acetate (PIN)
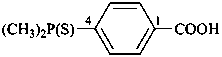
4-(dimethylphosphinothioyl)benzoic acid (PIN)
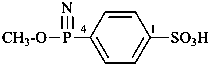
4-(methoxyphosphoronitridoyl)benzene-1-sulfonic acid (PIN)
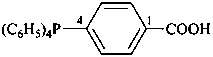
4-(tetraphenyl-λ5-phosphanyl)benzoic acid (PIN)
4-(tetraphenylphosphoranyl)benzoic acid
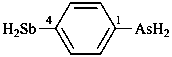
(4-stibanylphenyl)arsane (PIN)
(4-stibinophenyl)arsine
Bismuth compounds are named substitutively on the basis of parent hydrides named in accordance with the rules described in Chapter P-2. Suffixes and prefixes are used as indicated for substitutive nomenclature. There are no acids having retained names subject to functional class nomenclature. Substitutive nomenclature is preferred to functional nomenclature to designate oxides, sulfides, selenides, tellurides, and imides. Preferred and preselected names are chosen as for P, As, and Sb parents and prefixes.
BiH5
λ5-bismuthane (preselected name)
bismuthorane
H2Bi-BiH2
dibismuthane (preselected name)
H2Bi–
bismuthanyl (preselected prefix)
bismuthino
H3Bi=
λ5-bismuthanylidene (preselected prefix)
–HBi-BiH–
dibismuthane-1,2-diyl (preselected prefix)
Bi(CH3)3
trimethylbismuthane (PIN)
trimethylbismuthine
(C6H5)3Bi=O
triphenyl-λ5-bismuthanone (PIN)
triphenylbismuthane oxide
(C6H5)3Bi=NH
Bi.Bi,Bi-triphenyl-λ5-bismuthanimine (PIN)
triphenylbismuthane imide
bis(oxobismuthanyl) carbonate (PIN)
2,4-dioxa-1,5-dibismapentane-1,3,5-trione
2,7-dihydroxy-2H-1,3,2-benzodioxabismole-5-carboxylic acid (PIN)
(C6H5)3BiCl2 dichlorotri(phenyl)-λ5-bismuthane (PIN)
dichlorotri(phenyl)bismuthorane
2,2,2-triphenyl-1,3,2λ5-dioxabismetan-4-one (PIN)
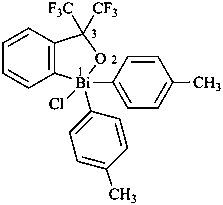
1-chloro-1,1-bis(4-methylphenyl)-3,3-bis(trifluoromethyl)-1,3-dihydro-2,1λ5-benzoxabismole (PIN)
P-68.4.0 IntroductionP-68.4.0 Introduction
P-68.4.1 Three or more homogeneous contiguous chalcogen atoms
P-68.4.2 Three or more heterogeneous contiguous chalcogen atoms
P-68.4.3 Chalcogen parent hydrides with nonstandard bonding numbers
The nomenclature of compounds containing chalcogen atoms depends on the number and kind of chalcogen atoms present. When one or two contiguous chalcogen atoms are present, chalcogen atoms are not used as parent hydrides, except for sulfones and disulfones when they are able to be expressed as described in previous sections. Hydroxy compounds, ethers, peroxols, peroxides, and their chalcogen analogues are described in P-63. However, when three or more contiguous identical chalcogen atoms are present these chalcogen atoms are always treated as parent hydrides in the normal way.
Sulfoxides and sulfones are also included in this type of nomenclature (see P-63.6).
P-68.4.1 Three or more homogeneous contiguous chalcogen atoms
P-68.4.1.1 Compounds with three or more contiguous identical chalcogen atoms are treated as parent hydrides in substitutive nomenclature.
Examples:
CH3-S-S-SH
methyltrisulfane (PIN)
CH3-O-O-O-CH3
dimethyltrioxidane (PIN)
dimethyl trioxide
CH3-S-S-S-CH3
dimethyltrisulfane (PIN)
dimethyl trisulfide
C6H5-Se-Se-Se-CH3
methyl(phenyl)triselane (PIN)
methyl phenyl triselenide
C6H5-Se-Se-Se-C6H5
diphenyltriselane (PIN)
diphenyl triselenide
(not triselanediyldibenzene)
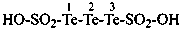
tritellanedisulfonic acid (preselected name)
tritelluropentathionic acid (traditional name)
HO-SO2-S-S-S-SO2-OH
trisulfanedisulfonic acid (preselected name)
pentathionic acid (traditional name)
CH3-SeSeSe-O-SH
methyltriselane-OS-thioperoxol (PIN)
O-(methyltriselanyl) thiohydroperoxide
H-TeTeTe-SeSe-H
tritellane(diselenoperoxol) (preselected name)
tritelluryl diselenohydroperoxide
C6H5-SSS-OH
phenyltrisulfanol (PIN)
CH3-TeTeTeTe-SH
methyltetratellanethiol (PIN)
–S-S-S–
trisulfanediyl (preselected prefix)
trithio
–Se-Se-Se-Se–
tetraselanediyl (preselected prefix)
tetraseleno
4,4′-triselanediyldibenzoic acid (PIN)
4,4′-triselenodibenzoic acid
Examples:
CH3-CH2-CO-S-S-S-CO-CH2-CH3
1,1′-trisulfanediyldi(propan-1-one) (PIN)
1,1′-trithiodi(propan-1-one)
CH3-CH2-CO-Se-Se-Se-CH3
1-(methyltriselanyl)propan-1-one (PIN; a pseudoketone)
(not Se-methyldiselanyl propaneselenoate, a pseudoester;
formation of
the pseudoester would require the fragmentation of a homogeneous chain)
1-(methyltriseleno)propan-1-one
CH3-CH2-CO-S-S-S-S-S-CO-CH3
1-(acetylpentasulfanyl)propan-1-one (PIN)
P-68.4.2.1 Compounds of the type a[ba]n are parent hydrides; they are discussed in P-21.2.3.1.
Examples:
CH3-S-O-SH
methyldithioxane (PIN)
(not methylsulfane-OS-thioperoxol)
CH3-S-O-S-CH3
dimethyldithioxane (PIN)
C6H5-S-O-S-CH3
methyl(phenyl)dithioxane (PIN)
HS-O-S-OH
dithioxanol (preselected name)
(sulfanyloxy)sulfanol
(not hydroxysulfane-OS-thioperoxol)
HO-S-O-S-OH
dithioxanediol (preselected name)
[not oxybis(sulfanol)]
Examples:
CH3-OO-SH
methyldioxidanethiol (PIN)
CH3-OOO-SH
methyltrioxidanethiol (PIN)
CH3-O-SOH
methyloxidane-SO-thioperoxol (PIN)
CH3-SS-OH
methyldisulfanol (PIN)
CH3-SSS-SeH
methyltrisulfaneselenol (PIN)
CH3-OO-SSH
methyldioxidanedithioperoxol (PIN)
CH3-O-S-SeSeH
methoxysulfanediselenoperoxol (PIN)
CH3-SS-OOH
methyldisulfaneperoxol (PIN)
CH3-S-O-S-OH
[(methylsulfanyl)oxy]sulfanol (PIN)
[not (methylsulfanyl)oxidane-SO-thioperoxol]
4,4′-diselenoxanediyldiphenol (PIN)
Examples:
H-OO-S-OH
(hydroperoxy)sulfanol (preselected name)
(not dioxidane-SO-peroxol)
H-OO-SS-H
disulfaneperoxol (preselected name)
[(not dioxidane(dithioperoxol)]
Examples:
CH3-OO-S-CH3
[(methylperoxy)sulfanyl]methane (PIN) [not methyl(methylsulfanyl)dioxidane]
(methyl-OOS-thiotrioxy)methane
CH3-S-S-O-CH2-CH3
[(methyldisulfanyl)oxy]ethane (PIN)
[methyl(dithioperoxy)oxy]ethane
[not ethoxy(methyl)disulfane]
(methyl-SSO-dithiotrioxy)ethane
C6H5-O-S-O-C6H5
1,1′-[sulfanediylbis(oxy)]dibenzene (PIN)
OSO-thiotrioxydibenzene
CH3-O-S-Se-C6H5
[(methoxysulfanyl)selanyl]benzene (PIN)
methyl-OSSe-selenothiotrioxybenzene
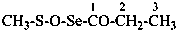
1-{[(methylsulfanyl)oxy]selanyl}propan-1-one (PIN)
1-[(acetylsulfanyl)peroxy]propan-1-one (PIN)
1,1′-[disulfanediylbis(oxy)]di(propan-1-one) (PIN)
1,1′-[dithioperoxybis(oxy)]di(propan-1-one)
3,6-dioxa-5-thia-4-selenanonan-7-one (PIN)
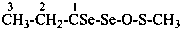
1-{[(methylsulfanyl)oxy]selanyl}propane-1-selone (PIN)
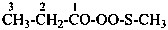
1-[(methylsulfanyl)peroxy]propan-1-one (PIN)
Many chalcogen compounds are designated by class names that were used as parent structures to name their derivatives. In these recommendations, in conformity with the principle that substitutive names are preferred, derivatives named on the basis of class names are retained for general use only.
P-68.4.3.1 Sulfanes, selanes, and tellanesP-68.4.3.1 Sulfanes, selanes, and tellanes
P-68.4.3.2 Di- and polysulfoxides, polysulfones, and selenium and tellurium analogues
P-68.4.3.3 Sulfimides, and chalcogen analogues, H2E=NH, where E = S, Se, or Te
P-68.4.3.4 Sulfinylamines, RN=E=O, sulfonylamines, RN=E(=O)2 and chalcogen analogues where E = S, Se, or Te
P-68.4.3.5 Sulfonediimines, RE(=NH)2R′ and chalcogen compounds where E = S, Se, or Te
P-68.4.3.6 Sulfoximides, R2E(=O)=NR′ and chalcogen analogues where E= S, Se, or Te
P-68.4.3.7 Sulfur diimides, HN=E=NH and chalcogen analogues where E = S, Se, or Te)
P-68.4.3.8 Sulfur triimides, E(=NH)3 and chalcogen analogues where E= S, Se, or Te)
Sulfanes, selanes, and tellanes that have a nonstandard bonding number are named on the basis of parent hydrides such as λ4-sulfane, λ6-sulfane, and λ4-selane, according to the λ-convention.
Examples:
(CH3-O)4S
tetramethoxy-λ4-sulfane (PIN)
[(trimethoxy-λ4-sulfanyl)oxy]methane
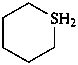
λ4-thiane (PIN)
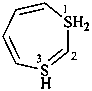
1H-1λ4,3λ4-dithiepine (PIN)
Compounds with the general structures R-[SO]n-R′ and R-[SO2]n-R′, in which n ≥ 2, have the class name ‘disulfoxides’, ‘trisulfoxides’, ‘disulfones’, etc. They and their selenium and tellurium analogues are named in two ways:
(1) substitutively by adding the suffix ‘one’ to the name of the appropriate parent hydride, λ4 or λ6 disulfane, diselane, ditellane, etc.;Method (1) generates preferred IUPAC names and is also used to name mixed sulfoxide-sulfones.(2) by functional class nomenclature, using class names such as ‘disulfoxide’, ‘disulfone’, ‘diselenoxide’, ‘diselenone’, etc.
Examples:
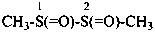
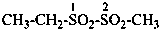
1-ethyl-2-methyl-1λ6,2λ6-disulfane-1,1,2,2-tetrone (PIN)
ethyl methyl disulfone
1-phenyl-2-(quinolin-7-yl)-1λ6,2λ6-diselane-1,1,2,2-tetrone (PIN)
phenyl quinolin-7-yl diselenone
diethyl-1λ6,2λ4-diselane-1,1,2-trione (PIN)
[(ethaneseleninyl)selenonyl]ethane
[(ethylseleninyl)selenonyl]ethane
Compounds with the general structures, H2S=NH, belong to a class called ‘sulfimides’ (CAS calls them sulfilimines). They are named in two ways:
(1) substitutively by adding the suffix ‘imine’ to the name of the parent hydride such as λ4-sulfane;Method (1) generates preferred IUPAC names.(2) substitutively using the class name ‘sulfimide’.
Example:
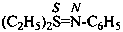
Example:
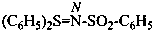
Compounds with the general structures, R-N=S=O and R-N=S(=O)2, have the class names ‘sulfinylamines’ and ‘sulfonylamines’, respectively. They are named substitutively on the basis of the parent hydrides λ4-sulfane or λ6-sulfane.
Examples:
CH3-N=S(=O)2
(methylimino)-λ6-sulfanedione (PIN)
(not N-sulfonylmethanamine)
(CH3)2S(O)=N-SO2-C6H5
N-[dimethyl(oxo)-λ6-sulfanylidene]benzenesulfonamide (PIN)
(benzenesulfonylimino)dimethyl-λ6-sulfanone
Compounds with the general structures, RE(=NH)2R′, have the class name ‘sulfonediimines’. Preferred IUPAC names are formed substitutively on the basis of the parent hydride λ6-sulfane. Names based on the class name ‘sulfonediimine’ are not recommended.
Example:
Compounds with the general structure R2E(=O)=NR′ have the class name ‘sulfoximides’ (CAS calls them sulfoximines). Preferred IUPAC names are formed substitutively on the basis of the parent hydride λ6-sulfane. Substitutive names may also be based on the functional parent name ‘sulfoximide’.
Example:
Compounds with the general structure, HN=S=NH, belong to a general class having the the class name ‘sulfur diimides’. Preferred IUPAC names are formed substitutively on the basis of the parent hydride λ4-sulfane. Functional class names are based on the class name ‘sulfur diimide’.
Example:
Compounds with the general structure, S(=NH)3, belong to a general class called ‘sulfur triimides’. Preferred IUPAC names are formed substitutively on the basis of the parent hydride λ6-sulfane. Functional class names are based on the class name ‘sulfur triimide’.
Example:
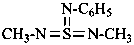
P-68.5.0 Substitutive nomenclatureP-68.5.0 Substitutive nomenclature
P-68.5.1 Nomenclature based on halogen parent hydrides
P-68.5.2 Nomenclature of halogen acids
P-68.5.3 Amides of halogen acids
In substitutive nomenclature, halogen atoms are denoted only by specific prefixes (see P-61.3), in functional class nomenclature by their class name (see P-15.2), and in functional replacement nomenclature by prefixes and infixes (see P-65.2.1.5).
Examples:
C6H5-ClO
chlorosylbenzene (PIN)
CH3-CH2-CO-Br
propanoyl bromide (PIN)
propionyl bromide
CH3-CH2-SO2-Cl
ethanesulfonyl chloride (PIN)
C6H5-P(O)Cl2
phenylphosphonic dichloride (PIN)
CH3-BCl2
dichloro(methyl)borane (PIN)
CH3P(Cl)(S-C2H5)
ethyl methylphosphonochloridothioite
(not ethyl methanephosphonochloridothioite)
For cyclic and acyclic parent hydrides the lambda convention is used to indicate nonstandard bonding numbers.
Examples:
CH3-ICl2
dichloro(methyl)-λ3-iodane (PIN)
(CH3-CO-O)2I–
bis(acetyloxy)-λ3-iodanyl (preferred prefix)
(not diacetoxyiodo)
(HO)2I–
dihydroxy-λ3-iodanyl (preselected name)
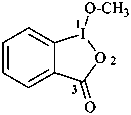
1-methoxy-1λ3,2-benziodoxol-3(1H)-one (PIN)
1H-1λ3-chlorole (PIN)
1λ3-bromirane (PIN)
1H-1λ3-benziodole (PIN)
The halogen acids HO-Cl, hypochlorous acid, HO-ClO, chlorous acid, HO-ClO2, chloric acid, and HO-ClO3, perchloric acid, and similar acids where Br, F, and I take the place of Cl, are discussed in P-67.1.1. They form esters (see P-67.1.3.2), such as ‘methyl chlorite’, CH3-O-ClO, and anhydrides (see P-67.1.3.3), such as ‘benzoic hypochlorous anhydride’, C6H5-CO-O-Cl.
Substituent groups derived from the halogen acids, such as chlorosyl, -ClO, bromyl, -BrO2, and periodyl, -IO3, are discussed in P-67.1.4.5 and exemplified in P-61.3.2.3.
P-68.5.3 Amides of halogen acids
The seniority order of classes (P-41) is applied to give preferred IUPAC names for amides of halogen acids.
Examples:
CH3-CH2-NH-Cl
ethylhypochlorous amide (PIN, see P-62.4)
N-chloroethanamine
P-69.0 IntroductionP-69.0 INTRODUCTION
P-69.1 Organometallic compounds involving the elements in Groups 13 through 16
P-69.2 Organometallic compounds involving the elements in Groups 3 through 12
P-69.3 Organometallic compounds involving the elements in Groups 1 and 2
P-69.4 Metallacycles
P-69.5 Seniority order for organometallic compounds
This Section is partly an application of the principles, rules, and conventions established in previous Chapters, and partly an extension of these principles, rules, and conventions to reconcile the nomenclatures of organic and inorganic compounds (see ref. 12) to name organometallic compounds.
Organometallic compounds are compounds having at least one bond between one metal atom and one carbon atom. In addition to the traditional metals, some compounds containing elements such as boron, arsenic, and selenium linked to a carbon atom have sometimes been considered to be organometallic.
Organometallic nomenclature in these recommendations is divided into three categories:
(1) organometallic compounds involving the elements of Groups 1 and 2;Thus, a great part of nomenclature of organometallic compounds is outside the scope of this book, which deals with organic compounds; it follows the principles, rules and conventions of the nomenclature of inorganic compounds described in the Nomenclature of Inorganic Chemistry (Red Book), in particular, Chapter IR-10 , ref. 12.(2) organometallic compounds involving the elements of Groups 3-12 (the transition metals); and
(3) organometallic compounds involving the elements of Groups 13 through 16.
All organometallic compounds can be named by a type of additive nomenclature conveniently called coordination nomenclature, a system in which the names of compounds are formed by adding the name(s) of (a) ligand(s), in alphanumerical order if there is more than one, to that of a central atom (ref. 12, Chapters IR-7, IR-9). However, organometallic compounds involving the elements of the Groups 13 through 16 can also be named by using substitutive nomenclature, as shown in Section P-68.
In this Section, all organometallic compounds are considered, first, those involving the elements of Groups 13 through 16 that are named substitutively, then those of the transition elements (Groups 3 through 12) and of Groups 1 and 2, that are named additively. Finally, the seniority rules necessary to name compounds that fall into both caregories are discussed.
Preferred IUPAC names are indicated for organometallic compounds named substitutively for the metals in Groups 14-16. However, neither preferred IUPAC names or preselected names (see P-12.2) for organometallic compounds involving the transition elements (including the Group 3 elements) and Groups 1 and 2 elements, except for ‘ocene’ compounds, are noted. This determination will await consideration by a task group on inorganic and/or organometallic nomenclature.
P-69.1 ORGANOMETALLIC COMPOUNDS INVOLVING THE ELEMENTS OF GROUPS 13 THROUGH 16
Organometallic compounds involving the elements of Groups 13, 14, 15, and 16 are named substitutively by prefixing the appropriate substituent names to the name of a parent hydride. Principles, rules, and conventions to name them have been discussed in Section P-68.
Examples:
Pb(CH2-CH3)4
tetraethylplumbane (PIN; substitutive name)
BrSb(CH=CH2)2
bromodi(ethenyl)stibane (PIN; substitutive name)
HIn(CH3)2
dimethylindigane (substitutive name)
P-69.2.1 Coordination nomenclature is the primary nomenclature method used to name organometallic compounds containing elements of Groups 3 through 12. It is described in Nomenclature of Inorganic Chemistry (IR-10, ref. 12). It is discussed briefly and exemplified in this Section.
P-69.2.2 Definitions of terms
In the rules that follow, certain terms are used in the sense indicated here. A ‘coordination entity’ refers to molecules or ions in which there is an atom (A) to which attached other atoms (B) or groups (C). The atom (A) is known as the ‘central atom’, and all other atoms which are directly attached to (A) are called ‘ligands’. Each central atom (A) has a ‘coordination number’ which is the number of atoms directly attached to it. A group containing more than one potential coordinating atom is termed a ‘multidentate’ ligand, the number of potential coordinating atoms being indicated by the terms ‘monodentate’, ‘bidentate’, etc. A ‘chelate’ ligand is a group attached to the central atom through two or more coordinating atoms, while a ‘bridging group’ is attached to more than one center of coordination. A ‘polynuclear’ coordination entity is one that contains more than one central atom, their number being designated by the terms ‘mononuclear’, ‘dinuclear’, etc., molecule.
Linear formulae are composed of the symbol of the central atom, followed by the ligands, in alphabetical order (as written) if more than one is present (IR-4.4.3.2, ref. 12). [This is a change from that described in the 1990 Inorganic Nomenclature recommendations.] In a line formula, a coordination entity is always placed in square brackets. No brackets are required when the structure is based on developed organic formulas. Abbreviations are used to represent complicated organic ligands in formulae (although they should not be used in names), for example Et = ethyl, ox = ethanedioato or oxalato, and py = pyridine; (see IR-4.4.3.2 and Tables VII and VIII, ref. 12).
In names of coordination compounds, the name of the central atom is placed after that of the ligand(s), which are listed in alphabetical order, regardless of the number of each. A compound or complex ligand is treated as a single unit. The names of anionic ligands, whether organic or inorganic, end in ‘o’. If the anion name ends in ‘ate’, ‘ite’, or ‘ide’, the final letter ‘e’ is replaced by ‘o’, giving ‘ato’, ‘ito’, or ‘ido’.
Examples:
C6H5–
benzenido
(CH3)2As–
dimethylarsanido
CH3-COO–
acetato (for the ending ‘ate’, see P-65.6.1)
Examples:
C6H5–
phenyl
(CH3)3Si–
trimethylsilyl
Examples:
CH3-NH2
methanamine
P-69.2.3 Compounds with at least one metal-carbon single bond
Compounds are named by citing the names of ligands, including hydrogen atom(s), in alphanumerical order, followed by the name of the metal. The presence of hydrogen attached to a metal atom must always be indicated by the prefix ‘hydrido’.
Examples:
[Pt{C(O)-CH3}(CH3)(PEt3)2]
acetyl(methanido)bis(triethylphosphane)platinum
acetyl(methyl)bis(triethylphosphane)platinum
[Os(CH2-CH3)(NH3)5]Cl
pentaammine(ethanido)osmium(1+) chloride
pentaammine(ethyl)osmium(1+) chloride
dihydrido(naphthalen-2-ido)rhenium
dihydrido(naphthalen-2-yl)rhenium
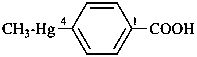
(4-carboxybenzenido)methanidomercury
(4-carboxyphenyl)(methyl)mercury
In order to indicate multicenter bonding to carbon atoms, for example in an unsaturated system, the name of the ligand is preceded by the prefix η (eta). A right superscript is added to the symbol η to indicate the number of atoms that bind to the metal. When it is necessary to indicate that not all unsaturation sites are bonded to the metal, numerical locants are added in front of the symbol η. It may also be necessary to denote a single atom in the ligand that is directly attached to the metal; in this case, the symbol κ (kappa) is cited before the element symbol that indicates the specific position that is bonded to the metal. See IR-10.2.5.1 and IR-10.2.3.3 (ref. 12) for a complete discussion on the use of η and κ symbols in additive nomenclature.
Examples:
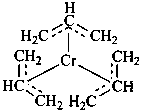
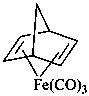
[(2,3,5,6-η)-bicyclo[2.2.1]hepta-2,5-diene]tricarbonyliron

dicarbonyl[(1−3-η)-cyclohepta-2,4,6-trien-1-ido](η5-cyclopenta-2,4-dien-1-ido)molybdenum
dicarbonyl[(1−3-η)-cyclohepta-2,4,6-trien-1-yl](η5-cyclopenta-2,4-dien-1-yl)molybdenum
dicarbonyl[(1−3-η)-cyclohepta-2,4,6-trien-1-ido](η5-cyclopentadienido)molybdenum

tricarbonyl(η7-cycloheptatrienylium)molybdenum(1+)
tricarbonyl(η7-cyclohepta-2,4,6-trien-1-ylium)molybdenum(1+)
The prefix ‘μ’ (see IR-9.2.5.2, ref. 12) is added at the front of the name of a bridging ligand to indicate bridging between two metal atoms. Locants for the ‘η’ positions are separated by a colon and a hyphen is added following the name of the bridging group. Direct bonding between metal atoms is indicated as described in IR-10.2.5.1 (ref. 12).
Example:
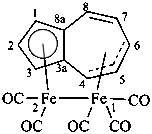
Organic molecules used as ligands are named substitutively in accordance with principles, rules. and conventions of substitutive nomenclature and cited in the name of the organometallic compound with the appropriate ‘η’ (hapto) symbols. This method is preferred to that consisting of using prefixes only to denote characteristic groups in the organic part of the organometallic compound.
Examples:


[(1,2,5,6-η)-cycloocta-1,5-diene][triphenyl(η6-phenyl)boranuido]rhodium
[(1,2,5,6-η)-cycloocta-1,5-diene][triphenyl(η6-phenyl)borato]rhodium
“Ocenes” are bis(η5-cyclopenta-2,4-dien-1-ido) or bis(η5-cyclopenta-2,4-dien-1-yl) or bis(η5-cyclopentadienido) complexes of certain metals. The names ferrocene, ruthenocene, osmocene, nickelocene, chromocene, cobaltocene, and vanadocene are names for compounds corresponding to ‘bis(λ5-cyclopenta-2,4-dien-1-yl)metal’, where the metal atom is Fe, Ru, Os, Ni, Cr, Co, and V. Ocenes are considered as heterocycles and seniority is based on the extension of the list of seniority for heterocycles through Groups 12, 11, 10, 9, 8, 7, 6, 5, 4, 3, 2, 1, for example nickelocene > cobaltocene > ferrocene. These names are substituted in accordance with the principles, rules, and conventions of substitutive nomenclature, using suffixes or prefixes to denote characteristic groups.
Examples:
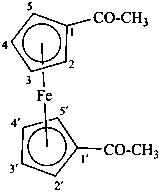
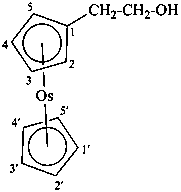
2-(osmocen-1-yl)ethanol (PIN)
1-(2-hydroxyethyl)osmocene
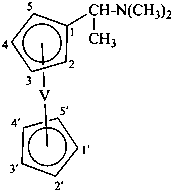
N,N-dimethyl-1-vanadocenylethan-1-amine (PIN)
[1-(dimethylamino)ethyl]vanadocene
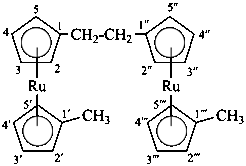
1,1′′-(ethane-1,2-diyl)bis(1′-methylruthenocene) (PIN)
1,3(1,1′)-diferrocenacyclotetraphane (PIN)
3,5-(ferrocene-1,1′-diyl)pentanoic acid (PIN)
1,1′-(4-carboxybutane-1,3-diyl)ferrocene
benzoferrocene (PIN)
(η5-cyclopenta-2,4-dien-1-ido)[(1,2,3,3a,7a)-η5-1H-inden-1-ido]iron
(η5-cyclopenta-2,4-dien-1-yl)[(1,2,3,3a,7a)-η5-1H-inden-1-yl]iron
(η5-cyclopentadienido)[(1,2,3,3a,7a)-η5-indenido]iron
Although many organometallic compounds of Groups 1 and 2 elements exist in associated molecular form and contain structural solvent, their names are based on the stoichiometric compositions of the compounds, the solvent, if any, being ignored. Additive names are formed by citing the names of the ligands denoting the organic groups and the ligand ‘hydrido’ denoting the hydrogen atoms, if any, in alphabetical order, followed by the name of the metal.
Examples:
NaCH=CH2 or[Na(CH=CH2)]
ethenidosodium
ethenylsodium
vinylsodium
(sodium ethenide is also acceptable;
it is a compositional name analogous to sodium chloride; see IR-5, ref. 12)
[LiMe]
methanidolithium
methyllithium
[(LiMe)4]
tetra-μ3-methanido-tetralithium
tetra-μ3-methyl-tetralithium
(LiMe)n
poly(methyllithium)
poly(methanidolithium)
LiMe
lithium methanide (compositional name; see IR-5, ref. 12)
[MgI(Me)]
iodido(methanido)magnesium
(additive name of coordination type)
[MgMe]I
methylmagnesium iodide (compositional name;
the formally electropositive component named by additive nomenclature)
[MgI(Me)]n
poly[iodido(methanido)magnesium], or
poly[iodido(methyl)magnesium]
MgIMe
magnesium iodide methanide
(compositional name, see IR-5, ref. 12)
Metallacycles are organic heterocycles in which one or more heteroatoms is (are) metal atoms other than the metals normally included in the nomenclature systems for heteromonocycles (see P-22.2). They may be named by extending the Hantzsch-Widman system to include the metallic elements in addition to those in Groups 13 through 16, but with a ‘0’ standard valence and the stems described in Table 2.5, for example ‘ine’ and ‘inane’ for unsaturated and saturated six-membered heterocycles, respectively or by selecting a parent hydrocarbon ring or ring system and replacing one or more carbon atoms by a metal atom from Groups 2 through 12 using a nondetachable skeletal replacement (‘a’) prefix to create the metallacyclic parent hydride. The name is adjusted to conform to the observed formula by substitutions using detachable prefixes on the ring and appropriate ligand names to describe atoms or groups attached to the metal atom.
The option to include the metallic elements in addition to the metals in Groups 13 through 16 in the Hantzsch-Widman system and their skeletal replacement (‘a’) prefixes is a major change from previous recommendations on the Hantzsch-Widman system.
Note: A project to consider the nomenclature for metallacycles containing transition metals has been established.
Examples:
2,2,2,2-tetracarbonyl-1,1-dichloro-1,2-silaferrolane
(Hantzsch-Widman type name)
2,2,2,2-tetracarbonyl-1,1-dichloro-1-sila-2-ferracyclopentane
(skeletal replacement name)
6,6-di(η5-cyclopenta-2,4-dien-1-ido)-6-titanabicyclo[3.2.0]heptane
(skeletal replacement name)
6,6-di(η5-cyclopentadienyl)-6-titanabicyclo[3.2.0]heptane
(skeletal replacement name)
6,6-di(η5-cyclopentadienido)-6-titanabicyclo[3.2.0]heptane
(skeletal replacement name)
1-carbonyl-3,5-dimethyl-1,1-bis(triethylphosphane)-1-iridabenzene
(skeletal replacement name)
9,9-[methylenebis(dimethylphosphane)]-10H-9-platinaanthracene
(skeletal replacement name)
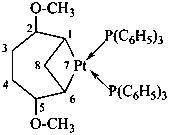
2,5-dimethoxy-7,7-bis(triphenylphosphane)-7-platinabicyclo[4.1.1]octane
(skeletal replacement name)
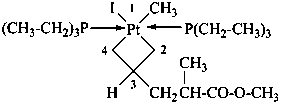
methyl 3-[1-iodo-1-methyl-1,1-bis(triethylphosphane)platinetan-3-yl]-2-methylpropanoate
(Hantzsch-Widman name)
methyl 3-[1-iodido-1-methanido-1,1-bis(triethylphosphane)platinetan-3-yl]-2-methylpropanoate
(Hantzsch-Widman name)
methyl 3-[1-iodo-1-methyl-1,1-bis(triethylphosphane)-1-platinacyclobutan-3-yl]-2-methylpropanoate
(skeletal replacement name)
When two different metals are present in an organometallic compound, one must be chosen as the basis of the name. Metals are classified into:
(1) metals of Groups 1 through 12; andP-69.5.1 Compounds having two identical or different metal atoms belonging to the first class are named additively using the methodology described in Section IR-9.2.5 of ref. 12, and the order of seniority of the Element Sequence Table beginning at Zn, i.e. Zn > Cd > Hg >.....Li > Na > K > Rb > Cs > Fr (see Table VI, ref. 12).(2) metals of Groups 13 through 16.
Example:
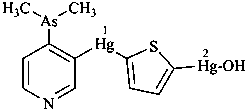
Example:
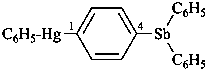
Examples:
H2Bi-GeH3
germylbismuthane
Pb(SnH3)4
plumbanetetrayltetrakis(stannane)
Return to:
Blue Book home page.