P-31.1 The endings ‘ene’ and ‘yne’
P-31.2 Parent hydrides modified by the prefix ‘hydro’ and ‘dehydro’
Division VIII Chemical Nomenclature and Structure Representation Division
P-30 Introduction
P-31 Modification of the degree of hydrogenation of parent hydrides
P-32 Substituent prefixes for substituents derived from parent hydrides with a modified degree of hydrogenation
P-33 Suffixes
P-34 Functional parent compounds
P-35 Prefixes corresponding to characteristic groups
The prefixes and/or suffixes attached to a parent name specify a particular molecular structure and usually represent substituents of various types, which are considered to take the place of hydrogen atoms of the parent hydride or parent structure. It has been customary to regard such substituents as characteristic (or functional) when the link between substituent and parent is not a carbon-carbon bond, for example, –OH, =O, and –NH2, but exceptions are recognized, such as –COOH and –CN. It seems appropriate to retain the general view of functionality as implying the presence of heteroatoms, but it would not be helpful to attempt to define precisely the limits of application of the term.
Carbon-carbon unsaturation in acyclic and alicyclic compounds is regarded by IUPAC as a special type of functionality and is therefore treated here in Chapter P-3 rather than in Chapter P-2 (Parent Hydrides). Its presence here and that of the hydrogenation of parent hydrides having the maximum number of noncumulative double bonds (mancude parent hydrides) is logical for nomenclature, as the unsaturation in acyclic and alicyclic parent hydrides expressed by endings and the saturation of mancude parent hydrides by hydro-dehydro prefixes are essentially equivalent.
This Chapter also deals with functional parent compounds, i.e., structures that may be treated as parent structures, having substitutable hydrogen atoms, but possessing characteristics normally associated with functionality, e.g. acetic acid, CH3-COOH, and phosphonic acid, HP(O)(OH)2. Functional parent compounds must be distinguished from compounds having a characteristic group systematically introduced as a suffix attached to a parent hydride, for example butanoic acid and ethanol. The latter compounds may be called ‘functionalized parent hydrides’, though strictly speaking, ions and radicals do not fall within the concept of functionality as described above; an ionic center or a radical center is treated like a function and expressed in the same way as characteristic groups, i.e., by suffixes and prefixes. This treatment is introduced in this Chapter and Chapter P-7.
Any group may be expressed in a name either as a suffix or as a prefix. As prefixes, they are detachable (alphabetizable), as are the prefixes derived from parent hydrides discussed in section P-14.5.
P-31 MODIFICATION OF THE DEGREE OF HYDROGENATION OF PARENT HYDRIDES
P-31.0 Introduction
P-31.1 The endings ‘ene’ and ‘yne’
P-31.2 Parent hydrides modified by the prefix ‘hydro’ and ‘dehydro’
P-31.0 INTRODUCTION
Parent hydrides are divided into two groups, fully saturated or fully unsaturated. Fully unsaturated cyclic parent hydrides are, by convention, defined as having the maximum number of noncumulative double bonds, also called ‘mancude’ compounds (an acronym for MAximum number of NonCUmulated DoublE bonds) . Thus a degree of hydrogenation different from those denoting these two groups must be expressed by an additive or subtractive operation corresponding to the addition or the subtraction of hydrogen atoms. Specific rules are devised for compounds having saturated and unsaturated parts, such as cyclophanes, spiro compounds, etc.
The state of hydrogenation of parent hydrides is modified in two ways: (a) by a subtractive operation (subtraction of two or more hydrogen atoms) denoted by the ‘ene’ and ‘yne’ endings or by the prefix ‘dehydro’; or (b) by an additive operation (addition of two or more hydrogen atoms) denoted by the prefix ‘hydro’.
In these recommendations, the prefixes ‘hydro’ and ‘dehydro’ are detachable, but are not included in the category of alphabetized detachable prefixes (see P-14.4; see also P-15.1.5.2, P-31.2, P-58.2). This is a change from the recommendations in earlier editions (ref. 1, 2). When along with the endings ‘ene’ and ‘yne’ they are used to modify parent hydrides, they are regulated by the principle of lowest locants, in accord with the numbering of the parent hydride and after priority has been given to indicated hydrogen, added indicated hydrogen, and suffixes, when present, as specified in the general rules for numbering (P-14.4).
P-31.1 THE ENDINGS ‘ENE’ OR ‘YNE’ ENDINGS
P-31.1.1 General methodology
P-31.1.1.1 The presence of one or more double or triple bonds in an otherwise saturated parent hydride [except for parents with Hantzsch-Widman names (see P-22.2.2.1.1), or retained names denoting partial hydrogenation as indicated later (see P-31.2.3.3.1)] is denoted by changing the ending ‘ane’ of the name of a saturated parent hydride to ‘ene’ or ‘yne’. Locants as low as possible are given to multiple bonds as a set, even though this may at times give ‘yne’ endings lower locants than ‘ene’ endings. If a choice remains, preference for low locants is given to the double bonds. In names, the ending ‘ene’ always precedes ‘yne’, with elision of the final letter ‘e’ in ‘ene’. Only the lower locant for a multiple bond is cited, except when the numerical difference between the two locants is greater than one, in which case the higher locant is enclosed in parentheses.
Exceptionally, the endings ‘ene‘ and ‘yne‘ can be added to the ending ‘ane’ in spiro compounds (see SP-2.4, ref. 8), phane nomenclature (see PhII-5.3.1, ref. 6), and ring assemblies (see P-31.1.7 in this book).
If the stereochemistry of a double bond is indicated in a structure it should be specified by E or Z with the appropriate locant. This includes double bonds in rings of eight or more members (see P-91.2.2).
Examples:
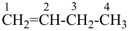
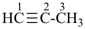
propyne (PIN)
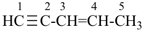
pent-3-en-1-yne (PIN)
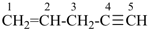
pent-1-en-4-yne (PIN)
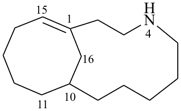
4-azabicyclo[8.5.1]hexadec-1(15)-ene (PIN)
Examples:
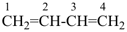
nona-1,3,5,7-tetraene (PIN)
P-31.1.2 Acyclic parent hydridesThis method is not used to modify Hantzsch-Widman names for saturated heterocyclic compounds or for totally or partially hydrogenated mancude compounds having retained names, i.e., imidazolidine, morpholine, piperazine, piperidine, pyrazolidine, pyrrolidine (see Table 2.3), quinuclidine (see Table 2.6), and also indane, chromane, isochromane, their chalcogen analogues, and indoline, isoindoline (see Table 3.1). When necessary, the corresponding mancude compounds are modified by using prefixes ‘hydro’ or ‘dehydro’, as indicated in P-31.2, below.
P-31.1.3 Monocyclic parent hydrides
P-31.1.4 Bi- and polycyclic von Baeyer parent hydrides
P-31.1.5 Spiro compounds
P-31.1.6 Phane parent hydrides
P-31.1.7 Ring assemblies of unsaturated components
P-31.1.2 Acyclic parent hydrides
P-31.1.2.1 Retained names
The name acetylene is retained for the compound HC≡CH. It is the preferred IUPAC name, but substitution of any kind is not allowed; however, in general nomenclature, substitution is allowed, for example fluoroacetylene [fluoroethyne (PIN)], but not by alkyl groups or any other group that extends the carbon chain, nor by characteristic groups expressed by suffixes.
The name allene, for CH2=C=CH2, is retained for general nomenclature only; substitution is allowed, but not by alkyl or any other group that extends the carbon chain, nor characteristic groups expressed by suffixes. The systematic name, propa-1,2-diene, is the preferred IUPAC name.
The name isoprene, for CH2=C(CH3)-CH=CH2, is retained but only for general nomenclature; no substitution of any kind is allowed. The systematic name, 2-methylbuta-1,3-diene, is the preferred IUPAC name.
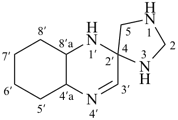
The name formazan, for 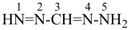 , is retained and is a preferred IUPAC name fully substitutable by suffixes and prefixes.
, is retained and is a preferred IUPAC name fully substitutable by suffixes and prefixes.
The name carbodiimide, for HN=C=NH, is retained but only for general nomenclature; no substitution of any kind is allowed. The systematic name, methanediimine, is the preferred IUPAC name.
P-31.1.2.2 Systematic names
P-31.1.2.2.1 Homogeneous acyclic parent hydrides and acyclic parent hydrides composed of alternating heteroatoms are modified by the general method of P-31.1.1.
Examples:
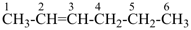
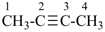
but-2-yne (PIN)
(not dimethylacetylene)
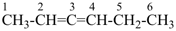
hexa-2,3-diene (PIN)
(not 1-ethyl-3-methylallene)
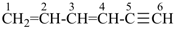
hexa-1,3-dien-5-yne (PIN)
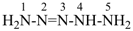
pentaaz-2-ene
(preselected name, see P-12.2)
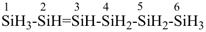
hexasil-2-ene
(preselected name, see P-12.2)
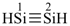
disilyne
(preselected name, see P-12.2)
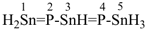
tristannaphospha-1,3-diene
(preselected name, see P-12.2)
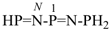
N-phosphanylidene-1-(phosphanylimino)phosphanamine
(preselected name, see P-12.2)
Locants are assigned to unsaturation sites in chains in accordance with the fixed numbering of the hetero chain. If a choice remains, then lowest locants are assigned to unsaturated sites.
Examples:
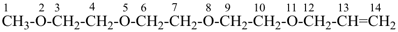
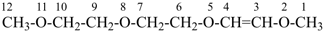
2,5,8,11-tetraoxadodec-3-ene (PIN)
P-31.1.3.1 In monocyclic homogeneous unsaturated compounds, one double or triple bond is always allocated the locant ‘1’. When alone, the locant ‘1’ is omitted in names.
Examples:
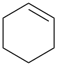
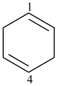
cyclohexa-1,4-diene (PIN)

cycloocta-1,3,5,7-tetraene (PIN)
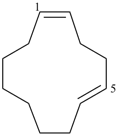
(1Z,5E)-cyclododeca-1,5-diene (PIN)
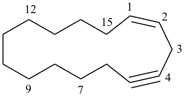
(1Z)-cyclopentadec-1-en-4-yne (PIN)
Examples:
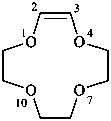

(3E)-1-oxa-4-azacyclododec-3-ene (PIN)
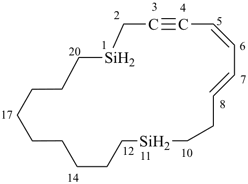
(5Z,7E)-1,11-disilacycloicosa-5,7-dien-3-yne (PIN)
(not 1,11-disilacycloicosa-4,6-dien-8-yne; the locant set ‘3,5,7’ is lower than ‘4,6,8’)
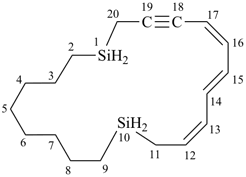
(12Z,14E,16Z)-1,10-disilacycloicosa-12,14,16-trien-18-yne (PIN)

1-azacyclotrideca-2,4,6,8,10,12-hexaene (PIN)
1H-1-aza[13]annulene
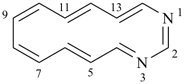
1,3-diazacyclotetradeca-1,3,5,7,9,11,13-heptaene (PIN)
1,3-diaza[14]annulene
Cyclic cumulenes are composed entirely of atoms, identical or different, linked by double bonds. For homocyclic cumulenes omission of all locants is recommended for preferred IUPAC names (see P-14.3.4.5).
Examples:
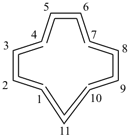
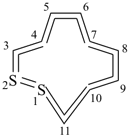
1λ4,2λ4-dithiacycloundecaundecaene (PIN)
Styrene, stilbene, and fulvene are the only retained names for parent hydrides containing a monocyclic ring system and side-chain unsaturation. All are used only in general nomenclature. Styrene and stilbene can be substituted only on the ring and only as prescribed in P-15.1.8.2.2; fulvene cannot be substituted at all.
Examples:
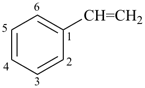
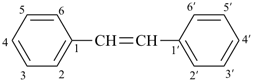
stilbene (ring substitution only)
1,1′-(ethene-1,2-diyl)dibenzene (PIN)
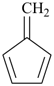
fulvene (no substitution)
5-methylidenecyclopenta-1,3-diene (PIN)
It should be noted that some bi- and polycyclic von Baeyer parent hydrides qualify for phane names as preferred IUPAC names (see P-52.2.5).
P-31.1.4.1 Low locants are allocated first in accordance with the fixed numbering of the ring system. Low locants are allocated for double bonds when the atoms of each bond have consecutive locants.
Examples:

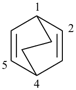
bicyclo[2.2.2]octa-2,5-diene (PIN)
(1) a minimum number of compound locants. A compound locant is used for a double bond if the locants of the atoms at each end of the bond do not differ by a value of one. When a compound locant is required, the higher locant is cited in parentheses. A benzene ring is shown and described as a cyclohexatriene corresponding to the Kekulé structure. Other aromatic rings are treated similarly, when required;
Note: The seniority of single locants over compound locants was established in 1989 for numbering steroids [see S3-2.5(2), ref. 16] and extended to von Baeyer systems in the 1999 publication on von Baeyer nomenclature (see VB-8.3.1, ref. 7).
Examples:
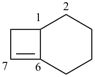
bicyclo[6.5.1]tetradec-8-ene (PIN)
[not bicyclo[6.5.1]tetradec-1(13)-ene]

bicyclo[12.2.2]octadeca-1(16),14,17-triene
[not bicyclo[12.2.2]octadeca-1(17),14(18),15-triene]
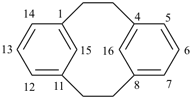
1(1,4)-benzenacyclotridecaphane (PIN, see P-52.2.5)
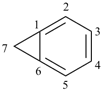
bicyclo[4.1.0]hepta-1,3,5-triene (PIN)
[not bicyclo[4.1.0]hepta-1(6),2,4-triene]
Examples:
 | not |  | |
| (I) | (II) | ||
| nor |  | nor |  |
| (III) | (IV) | ||
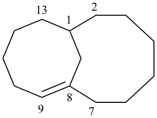
| tricyclo[9.3.1.14,8]hexadeca-1(15),11,13-triene (I) [not tricyclo[9.3.1.14,8]hexadeca-4(16),5,7-triene (II); nor tricyclo[9.3.1.14,8]hexadeca-4,6,8(16)-triene (III); nor tricyclo[9.3.1.14,8]hexadeca-1(14),11(15),12-triene (IV); the set of locants ‘1,11,13’ in (I) is lower than ‘4,5,7’ in (II) or ‘4,6,8’ in (III); and (IV) has two compound locants whereas (I) has only one compound locant] 1(1,3)-benzena-4(1,3)-cyclohexanacyclohexaphane (PIN, see P-52.2.5) | |||
 | not | 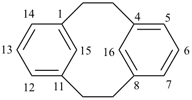 | |
| (I) | (II) | ||
| nor | 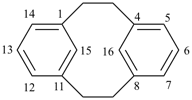 | nor | 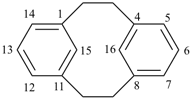 |
| (III) | (IV) | ||
| tricyclo[9.3.1.14,8]hexadeca-1(15),4(16),5,7,11,13-hexaene (I) not tricyclo[9.3.1.14,8]hexadeca-1(15),4,6,8(16),11,13-hexaene (II); nor tricyclo[9.3.1.14,8]hexadeca-1(14),4,6,8(16),11(15),12-hexaene (III); nor tricyclo[9.3.1.14,8]hexadeca-1(14),4(16),5,7,11(15),12-hexaene (IV) the set of locants in (I) ‘1,4,5,7,11,13’ is lower than ‘1,4,6,8,11,13’ in II); also name (I) has two compound locants compared to three in names (III) and IV)] 1,4(1,3)-dibenzenacyclohexaphane (PIN, see P-52.2.5) | |||
Example:
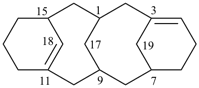
(1) compound locants are kept to a minimum.
Example:
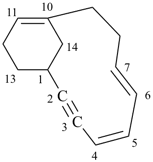
Example:
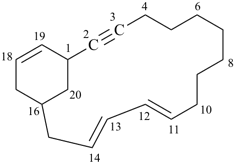
Example:
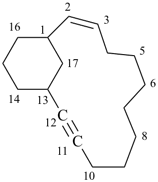
Examples:
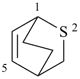
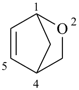
2-oxabicyclo[2.2.1]hept-5-ene (PIN)
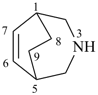
3-azabicyclo[3.2.2]non-6-ene (PIN)
P-31.1.5.1 Spiro compounds composed of unsaturated rings
P-31.1.5.1.1 Low locants are assigned to double bonds in accordance with the fixed numbering of the spiro compound.
Examples:
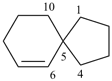
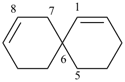
spiro[5.5]undeca-1,8-diene (PIN)
(1) lower locants are assigned to multiple bonds as a set;
Example:
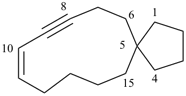
Example:
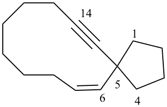
Examples:
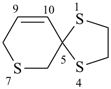
1,4,7-trithiaspiro[4.5]dec-9-ene (PIN)
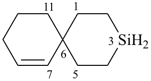
3-silaspiro[5.5]undec-7-ene (PIN)
P-31.1.5.2.1 Unsaturation in a saturated spiro ring system with components named by the von Baeyer system is indicated by the endings ‘ene’ and the multiplicative prefixes ‘di’, ‘tri’, etc. when required; the ending ‘ene’ is cited after the last bracket of the spiro name. The final letter ‘e’ of the saturated hydrocarbon name is elided if followed by a vowel. If there is a choice, low locants are assigned, in order, to spiro junction(s), heteroatoms and double bonds (see SP-2.4 in ref. 8).
Note: The placement location of the ‘ene’ endings after the closing brackets around the parent spiro name was established in the publication on nomenclature of spiro compounds ( ref. 8)Examples:
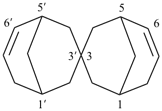
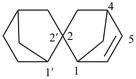
2,2′-spirobi[bicyclo[2.2.1]heptan]-5-ene (PIN)
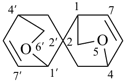
5,6′-dioxa-2,2′-spirobi[bicyclo[2.2.2]octane]-7,7′-diene (PIN)

2-phospha-3,3′-spirobi[bicyclo[3.3.1]nonane]-6′,7-diene (PIN)
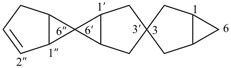
3,3′:6′,6′′-dispiroter[bicyclo[3.1.0]hexan]-2′′-ene (PIN)
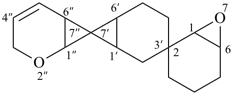
2′′,7-dioxa-2,3′:7′,7′′-dispiroter[bicyclo[4.1.0]heptan]-4′′-ene (PIN)
[not 5′′,7-dioxa-2,3′:7′,7′′-dispiroter[bicyclo[4.1.0]heptan]-2′′-ene;
the locant set ‘2′′,7’ is lower than ‘5′′,7’ for the ‘oxa’ prefixes]
P-31.1.6.1 Double bonds in amplificants and in simplified phane skeletons
The presence of one or more double or triple bonds in an otherwise saturated phane parent hydride, except in amplificants with Hantzsch-Widman names, is denoted by changing the final letter ‘e’ of the phane parent hydride name to ‘ene’ or ‘yne’, with appropriate multiplying prefixes to indicate the multiplicity of each kind of unsaturated site (ref. 6, PhII-5.3).
Low locants are allocated for double or triple bonds in accordance with the fixed numbering of the phane parent hydride and of phane parent hydrides modified by skeletal replacement (‘a’) nomenclature. Three types of locants are used to fully describe compounds derived from phane parent hydrides:
(1) primary locants, i.e. arabic number locants that denote the atoms and superatoms of the phane parent skeleton;
(2) composite locants, i.e. primary locants with a superscript arabic number locant denoting positions in amplificants (see P-26.4.3);
(3) compound locants, which are primary or composite locants followed by another locant in parentheses, indicating that a double bond is not located between two consecutive locants.
In phane nomenclature, double and triple bonds are denoted in two ways:
(1) by the lowest locant of a double or triple bond when two consecutive locants are:
(a) primary locants;(2) by a compound locant, when two locant are not consecutive, or when one is a primary locant and the other a composuit locant.
If there is a choice the criteria of P-31.1.4.3 are applied.
This is a change from PhII-5.2.3.
Examples:
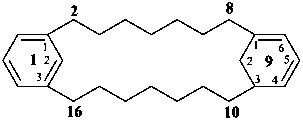
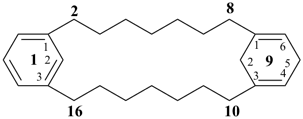
1(1,3)-benzena-9(1,3)-cyclohexanacyclohexadecaphane-91(96),93-diene (PIN)
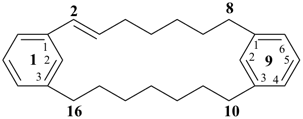
1,9(1,3)-dibenzenacyclohexadecaphan-2-ene (PIN)
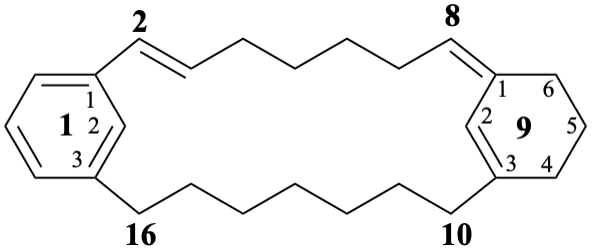
(2E,8(91)Z)-1(1,3)-benzena-9(1,3)-cyclohexanacyclohexadecaphane-2,8(91),92-triene
Double and triple bonds in a phane structure are described by the method of P-31.1.4.3. Low locants are allocated to double and triple bonds first when considered together as a set in ascending order and, if a choice is still needed, to double bonds.
Examples:
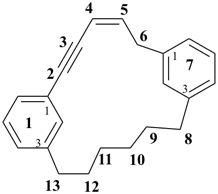

(2E)-1,7(1,3)-dibenzenacyclotridecaphan-2-en-5-yne (PIN)
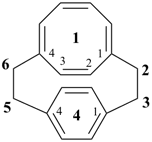
1(1,4)-cyclooctana-4(1,4)-benzenacyclohexaphane-11(18),12,14,16-tetraene (PIN)
P-31.1.7.1 Unsaturation in ring assemblies composed of saturated components, monocyclic or alicyclic, is indicated by the endings ‘ene’, ‘yne’, etc. and the multiplicative prefixes ‘di’, ‘tri’, etc; the endings are cited after the last bracket of the ring assembly name. The final letter ‘e’ of the saturated hydrocarbon name is elided if followed by a vowel. If there is a choice, low locants are assigned, in order, to ring assembly junction(s), heteroatoms and multiple bonds (see also P-31.1.5.2).
The location of the ‘ene’, ‘yne’, etc., endings after the closing brackets of the ring assembly name is a change from the 1979 and the 1993 Guide editions (ref. 1, 2) and is completely consistent with the method established for spiro compounds in the 1999 publication on nomenclature of spiro compounds (ref. 8)
Examples:
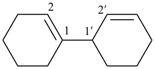

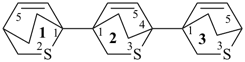
[2,2′-bi(bicyclo[2.2.2]octane)]-5,5′-diene (PIN)

[11,21:24,31-tercyclohexane]-12,21-diene (PIN)
[1,1′:4′,1′′-tercyclohexane]-1′,2-diene
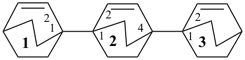
[11,21:24,31-terbicyclo[2.2.2]octane]-12,22,32-triene (PIN)
[1,1′:4′,1′′-terbicyclo[2.2.2]octane]-2,2′,2′′-triene
Examples:


[11,21:24,31-tercyclohexane]-11(21),24(31)-diene (PIN)
[1,1′:4′,1′′-tercyclohexane]-1(1′),4′(1′′)-diene
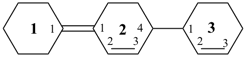
[11,21:24,31-tercyclohexane]-11(21),22,32-triene (PIN)
[1,1′:4′,1′′-tercyclohexane]-1(1′),2′,2′′-triene
Example:
P-31.2.1 The prefixes ‘hydro/dehydro’ are used to indicate addition and subtraction, respectively, of hydrogen atoms to or from mancude compounds. ‘Hydro’ and ‘dehydro’ prefixes are detachable prefixes but are not included in those prefixes that are cited in alphanumerical order. Thus, in names, they are cited immediately at the front of the name of the parent hydride, after alphabetized prefixes and before nondetachable prefixes.
If both hydro and dehydro prefixes are used in name, dehydro prefixes are cited before hydro prefixes with the order not affected by multipliers.
This is a change from previous recommendations. In these recommendations the prefixes ‘hydro’ and ‘dehydro’ are detachable but not alphabetized with other substituent prefixes. In names, they are cited immediately before the name of the parent hydride, after alphabetized prefixes and before nondetachable prefixes.
The starting point and direction of numbering of a compound are chosen so as to give lowest locants to fixed numbering of a polycyclic ring system, as in naphthalene, quinoline, etc., then to heteroatoms in heterocycles, and then to indicated hydrogen, when present, in accordance with the methodology described in P-14.4 for the construction of substitutive names.
Example:

‘Hydro’ and ‘dehydro’ prefixes are associated with hydrogenation and dehydrogenation, respectively, of a double bond; thus, multiplying prefixes of even values, as ‘di’, ‘tetra’, etc. are used to indicate the saturation of double bond(s), for example ‘dihydro’, ‘tetrahydro’; or creation of double (or triple) bonds, as ‘didehydro’, etc.. In names, they are placed immediately at the front of the name of the parent hydride and in front of any nondetachable prefixes. Indicated hydrogen atoms have priority over ‘hydro‘ prefixes for low locants. If indicated hydrogen atoms are present in a name, the ‘hydro‘ prefixes precede them.
4,5-dihydro-3H-azepine (PIN)
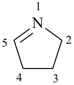
3,4-dihydro-2H-pyrrole (PIN)
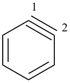
1,2-didehydrobenzene (PIN, see P-31.2.4.1)
cyclohex-1,3-dien-5-yne
(not benzyne)
P-31.2.3.1 ‘Hydro’ prefixes are used to modify the degree of hydrogenation of monocyclic mancude compounds having retained or systematic names, except for the hydro derivatives of benzene, for which the traditional names ‘cyclohexene’ and ‘cyclohexadiene’ are the preferred IUPAC names.
Examples:
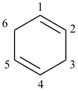
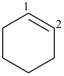
cyclohexene (PIN)
(not 1,2,3,4-tetrahydrobenzene)
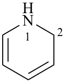
1,2-dihydropyridine (PIN)
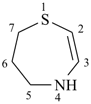
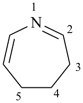
4,5,6,7-tetrahydro-1,4-thiazepine (PIN)
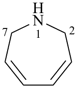
2,7-dihydro-1H-azepine (PIN)
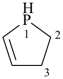
2,3-dihydro-1H-phosphole (PIN)
Preferred IUPAC names of saturated heteromonocyclic compounds are either Hantzsch-Widman names described in P-22.2.2.1.1 or retained names described in Table 2.3. Names of saturated rings derived by using hydro prefixes from Hantzsch-Widman names, retained names modified by adding the maximum of hydro prefixes, or ‘cyclo’ names described in P-22.2.5 are not preferred IUPAC names, but they may be used in general nomenclature.
Examples:
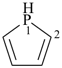
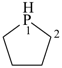
phospholane (PIN)
tetrahydro-1H-phosphole
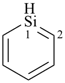
siline (PIN)
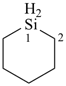
silinane (PIN)
hexahydrosiline
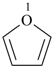
furan (PIN)
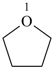
oxolane (PIN)
tetrahydrofuran
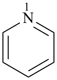
pyridine (PIN)
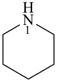
piperidine (PIN)
hexahydropyridine
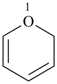
2H-pyran (PIN)
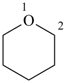
oxane (PIN)
tetrahydropyran

1,4-thiazepine (PIN)
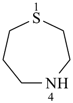
1,4-thiazepane (PIN)
hexahydro-1,4-thiazepine
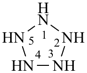
cyclopentaazane
pentazolidine (preselected name;
see P-12.1; P-22.2.2.1.5.2; P-22.2.5)
P-31.2.3.3.1 Retained names of partially saturated polycyclic mancude compoundsP-31.2.3.3.1 Retained names of partially saturated polycyclic mancude compounds
P-31.2.3.3.2 Polycyclic mancude compounds
P-31.2.3.3.3 Spiro compounds
P-31.2.3.3.4 Phane compounds
P-31.2.3.3.5 Ring assemblies
Names listed in Table 3.1 are retained names that are not used as preferred IUPAC names. They are, however acceptable in general nomenclature with full substitution, including characteristic groups expressed as suffixes, but not as fusion components nor as amplificants in Phane Nomenclature.
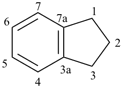
indane
(formerly indan)
2,3-dihydro-1H-indene (PIN)
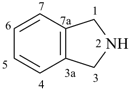
1H-indoline
2,3-dihydro-1H-indole (PIN)
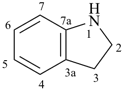
2H-isoindoline
2,3-dihydro-1H-isoindole (PIN)
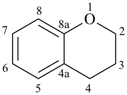
chromane
3,4-dihydro-2H-1-benzopyran (PIN)
3,4-dihydro-2H-chromene
thiochromane (S instead of O)
3,4-dihydro-2H-1-benzothiopyran (PIN)
3,4-dihydro-2H-thiochromene
selenochromane (Se instead of O)
3,4-dihydro-2H-1-benzoselenopyran (PIN)
3,4-dihydro-2H-selenochromene
tellurochromane (Te instead of O)
3,4-dihydro-2H-1-benzotelluropyran (PIN)
3,4-dihydro-2H-tellurochromene

isochromane
3,4-dihydro-1H-2-benzopyran (PIN)
3,4-dihydro-1H-isochromene
isothiochromane (S instead of O)
3,4-dihydro-1H-2-benzothiopyran (PIN)
3,4-dihydro-1H-isothiochromene
isoselenochromane (Se instead of O)
3,4-dihydro-1H-2-benzoselenopyran (PIN)
3,4-dihydro-1H-isoselenochromene
isotellurochromane (Te instead of O)
3,4-dihydro-1H-2-benzotelluropyran (PIN)
3,4-dihydro-1H-isotellurochromene
The degree of hydrogenation of partially or fully saturated individual mancude ring systems, carbocyclic or heterocyclic, is given by ‘hydro’ prefixes, in accordance with the general methodology described in P-31.2.2. Total hydrogenation is indicated by the appropriate multiplicative prefixes indicating the total number of hydrogen atoms attached, but locants are omitted (see P-14.3.4.5).
Examples:
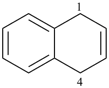
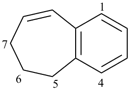
6,7-dihydro-5H-benzo[7]annulene (PIN)
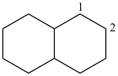
decahydronaphthalene (PIN)
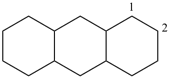
tetradecahydroanthracene (PIN)
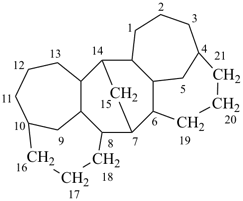
octadecahydro-7,14-methano-4,6:8,10-dipropanodicyclohepta[a,d][8]annulene (PIN)
hexacyclo[15.3.2.23,7.12,12.013,21.011,25]pentacosane (see P-23.2.6.3)
Spiro compounds including mancude components are modified in accordance with the general methodology described in P-31.2.2.
Examples:
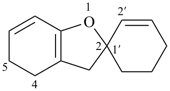
4,5-dihydro-3H-spiro[[1]benzofuran-2,1′-cyclohexan]-2′-ene (PIN)
When the name of an amplificant implies the presence of a maximum number of noncumulated double bonds, other states of hydrogenation are indicated by use of the prefix ‘hydro’. This method is applied as follows (see ref. 6, PhII-5.1 and PhII-5.2):
(1) ‘Hydro’ prefixes are used to modify mancude heteromonocycles having retained names or named in accordance with the extended Hantzsch-Widman system. However, names for the fully saturated heteromonocycles that have retained names or Hantzsch-Widman names are preferred to those expressed by ‘hydro’ prefixes, for example, oxolane and piperidine are preferred to tetrahydrofuran and hexahydropyridine, respectively.
(2) ‘Hydro’ prefixes are used to indicate all modifications of the degree of unsaturation of carbocyclic or heterocyclic mancude parent hydrides, except for benzene. Retained names of partially hydrogenated parent hydrides, such as indane and chromane (see P-31.2.3.3.1), are not recommended as amplificants in phane nomenclature.
Examples:
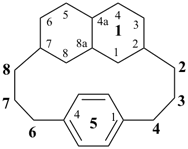
11,14-dihydro-1,7(2,6)-dipyridinacyclododecaphane (PIN)
The degree of hydrogenation of ring assemblies composed of mancude components is given by ‘hydro’ prefixes, in accordance with the general methodology described in P-31.2.2. Since assemblies are considered as parent hydrides, the degree of hydrogenation of the different components can be modified, to a certain extent, in a way that is not allowed for the individual components. This is the case of assemblies composed of monocyclic components, in which the names are different for the mancude and the saturated component. Thus, assemblies composed of monocyclic components and those composed of ring system components are treated differently.
P-31.2.3.3.5.1 Ring assemblies composed of monocyclic componentsP-31.2.3.3.5.1 Ring assemblies composed of monocyclic components
P-31.2.3.3.5.2 Ring assemblies composed of ring systems
(a) Ring assemblies composed of monocyclic mancude or saturated hydrocarbons. Low locants are assigned to ‘hydro’ prefixes in accordance with the fixed numbering of each assembly. In biphenyl and polyphenyl assemblies, one benzene ring must remain in the assembly; otherwise, the starting parent hydride is the saturated assembly and the ending ‘ene’ is used to denote unsaturation (see P-31.1.7). Furthermore, when a modified ring assembly of two rings consists of a benzene ring and a cyclohexane ring substitutive nomenclature is preferred (see P-54.3).
Examples:
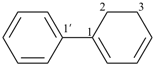
cyclohexylbenzene (PIN)
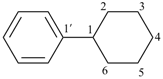
1,2,3,4,5,6-hexahydro-1,1′-biphenyl (numbering shown)
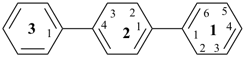
12,15,22,23-tetrahydro-11,21:24,31-terphenyl (PIN)
2,2′,3′,5-tetrahydro-1,1′:4′,1′′-terphenyl
[4-(cyclohexa-1,4-dien-1-yl)cyclohexa-1,3-dien-1-yl]benzene
(substitutive name)
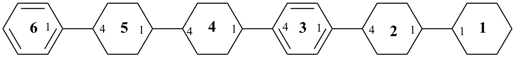
11,12,13,14,15,16,21,22,23,24,25,26,41,42,43,44,45,46,51,52,53,54,55,56-tetracosahydro-11,21:24,31:34,41:44,51:54,61-sexiphenyl (PIN)
1,1′,1′′′,1′′′ ′,2,2′,2′′′,2′′′ ′,3,3′,3′′′,3′′′ ′,4,4′,4′′′,4′′′ ′,5,5′,5′′′,5′′′ ′,6,6′,6′′′,6′′′ ′-tetracosahydro-1,1′:4′,1′′:4′′,1′′′:4′′′ ′1′′′ ′:4′′′ ′,1′′′ ′′-sexiphenyl
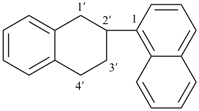
4-[4-([1,1′-bi(cyclohexan)]-4-yl)phenyl]-4′-phenyl-1,1′-bi(cyclohexane) (substitutive name)
{not 1-(21,22,23,24,25,26,31,32,33,34,35,36-dodecahydro[11,21:24,31-terphenyl]-14-yl)-4-([1,1′-bi(cyclohexan)]-4-yl)benzene (substitutive name)}
Note: According to P-52.2.5.1, seven nodes are required for a phane name.

1(1),4(1,4)-dibenzena-2,3,5,6(1,4),7(1)-pentacyclohexanaheptaphane (PIN; numbering shown)
4-[4-(4′-phenyl[1,1′-bi(cyclohexan)]-4-yl)phenyl)-11,21:24,31-tercyclohexane (substitutive name)
4-[4-(4′-phenyl[1,1-bi(cyclohexan)]-4-yl)pheny]-1,1′:4′,1′′-tercyclohexane (substitutive name)
Examples:

Low locants are assigned to the junctions between components, then to indicated hydrogen atoms, if any, and finally to ‘hydro’ prefixes.
Examples:

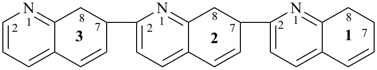
17,18,27,28,37,38-hexahydro-12,27:22,37-terquinoline (PIN)
7,7′,7′′,8,8′,8′′-hexahydro-2,7′:2′,7′′-terquinoline
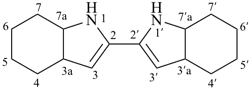
3a,3′a,4,4′,5,5′,6,6′,7,7′,7a,7′a-dodecahydro-1H,1′H-2,2′-biindole (PIN)
P-31.2.4.1 The subtractive prefix ‘dehydro’ is used to denote the removal of hydrogen atoms and the formation of multiple bonds. Its use is very limited in systematic nomenclature of organic compounds. Applied to benzene, it leads to the name ‘1,2-didehydrobenzene’, the preferred IUPAC name, rather than ‘benzyne’ that was formerly used. Applied to annulenes, it leads to didehydro[n]annulenes that are not used in preferred IUPAC names, but are acceptable for use in general nomenclature.
Examples:
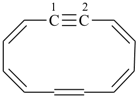
1,2-didehydro[12]annulene (numbering shown)
cyclododeca-1,3,5,7,9-pentaen-11-yne (PIN)
P-31.2.4.3 The use of the ‘dehydro’ prefix is not recommended to denote double bond unsaturation in heterocyclic rings having Hantzsch-Widman names. Names are formed preferably by using the ‘hydro’ prefix, as shown in the following examples.
Example:
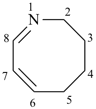 | not | 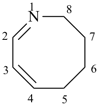 |
| (6Z,8Z)-2,3,4,5-tetrahydroazocine (PIN) | 1,2,3,4-tetradehydroazocane |
P-32.0 IntroductionP-32.0 INTRODUCTION
P-32.1 Substituent groups derived from parent hydrides with ‘ene’ or ‘yne’ endings
P-32.2 Substituent groups modified by the prefix ‘hydro’ and ‘dehydro’
P-32.3 Retained names for substituent groups derived from unsaturated acyclic parent hydrides
P-32.4 Retained names for substituent groups derived from partially saturated polycyclic parent hydrides
Names of substituents derived from the names of the corresponding unsaturated compounds described in Section P-31 are formed by using the appropriate suffixes ‘yl’, ‘ylidene’ or ‘ylidyne’, as described for the formation of substituent prefixes in Section P-29. These names of substituents may contain the endings ‘ene’ or ‘yne’, or the prefixes ‘hydro’ or ‘dehydro’ in the case of mancude compounds.
P-32.1 SUBSTITUENT GROUPS DERIVED FROM PARENT HYDRIDES WITH ‘ENE’ OR ‘YNE’ ENDINGS
P-32.1.1 Substituents derived from unsaturated acyclic compounds are named in two ways.
(1) As suffixes have priority for low locants, the position(s) of multiple bonds must be selected in accord with low locants assigned to free valences. These free valences can be in any position of modified parent structure(s). Accordingly, for acyclic parent structures, all locants for the free valences, including ‘1’, must be cited.
(2) Names can also be formed by substituting simple substituents into larger ones, in a manner similar to saturated prefixes described in Section P-29.
A major change in the naming of substituents derived from unsaturated acyclic compounds is adopted in these recommendations. The longest chain is chosen as the parent chain in preference to the number or type of multiple bonds.
For the method used in the formation of preferred IUPAC names, see P-57.1.1.1.
Examples:
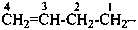
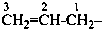
(1) prop-2-en-1-yl (preferred prefix)
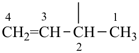 | 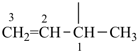 |
| (1) but-3-en-2-yl (preferred prefix) | (2) 1-methylprop-2-en-1-yl |
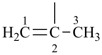 | 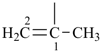 |
| (1) prop-1-en-2-yl (preferred prefix) isopropenyl (retained name, but not substitutable, see P-32.3) | (2) 1-methylethen-1-yl |
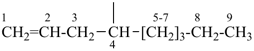 | 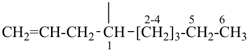 |
| (1) non-1-en-4-yl (preferred prefix) | (2) 1-(prop-2-en-1-yl)hexyl (not 1-pentylbut-3-en-1-yl) |
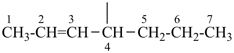 | 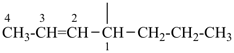 |
| (1) hept-2-en-4-yl (preferred prefix) | (2) 1-propylbut-2-en-1-yl |
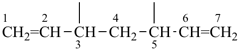 | 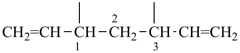 |
| (1) hepta-1,6-diene-3,5-diyl (preferred prefix) | (2) 1,3-diethenylpropane-1,3-diyl |
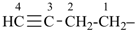
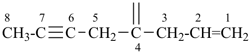 | 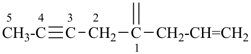 |
| (1) oct-1-en-6-yn-4-ylidene (preferred prefix) | (2) 1-(prop-2-en-1-yl)pent-3-yn-1-ylidene |
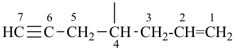 | 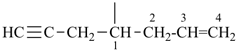 |
| (1) hept-1-en-6-yn-4-yl (preferred prefix) | (2) 1-(prop-2-yn-1-yl)but-3-en-1-yl |
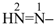
(1) diazenyl (preselected prefix; see P-12.2)
–N=N–
(1) diazenediyl (preselected prefix, see P-12.2)
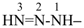
(1) triaz-2-en-1-yl (preselected prefix, see P-12.2)
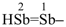
(1) distibenyl (preselected prefix; see P-12.2)
Method (1) described in P-32.1.1 is used to name monocyclic substituent groups.
Examples:
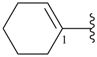
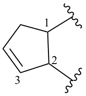
cyclopent-3-ene-1,2-diyl (preferred prefix)
Lowest possible locants are assigned first to free valence(s), then to unsaturated sites, in accordance with the fixed numbering of the parent hydride.
Examples:
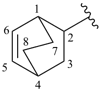
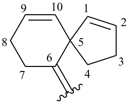
spiro[4.5]deca-1,9-dien-6-ylidene (preferred prefix)
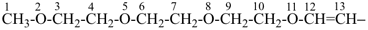
2,5,8,11-tetraoxatridec-12-en-13-yl (preferred prefix)
(not 3,6,9,12-tetraoxatridec-1-en-1-yl;
the suffix ‘-yl’ is added to the parent hydride name 2,5,8,11-tetraoxatridecane)
Names of partially unsaturated substituent groups derived from mancude compounds are formed by using the prefix ‘hydro’. When a choice is possible, low locants are assigned in accord with the order of seniority (see P-44.2 and also P-59.1.10) for assigning lowest possible locants to several nomenclature features. Indicated and added indicated hydrogen atoms must be cited in names.
P-32.2.1 For heteromonocyclic parent hydrides, when there is a choice heteroatoms have the lower possible locants, then indicated hydrogen atoms, followed by free valence suffixes and finally ‘hydro’ prefixes.
Examples:
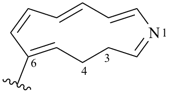
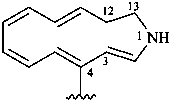
12,13-dihydro-1H-1-aza[13]annulen-4-yl
1-azacyclotrideca-2,4,6,8,10-pentaen-4-yl (preferred prefix)
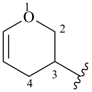
3,4-dihydro-2H-pyran-3-yl
(preferred prefix)
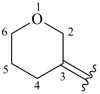
dihydro-2H-pyran-3(4H)-ylidene
oxan-3-ylidene (preferred prefix)
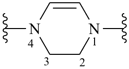
2,3-dihydropyrazine-1,4-diyl (preferred prefix)
Examples:
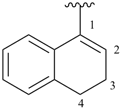
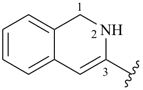
1,2-dihydroisoquinolin-3-yl (preferred prefix)
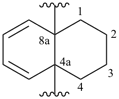
1,2,3,4-tetrahydronaphthalene-4a,8a-diyl (preferred prefix)
Examples:
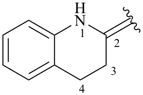
3,4-dihydroquinolin-2(1H)-ylidene (preferred prefix)
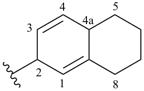
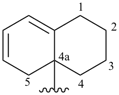
5,6,7,8-tetrahydronaphthalen-2(4aH)-ylidene (preferred prefix)
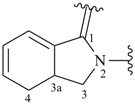
3a,4-dihydro-1H-isoindol-2(3H)-yl-1-ylidene (preferred prefix)
The names vinyl, for CH2=CH–; vinylidene, for CH2=C= ; allyl, for 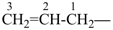 allylidene, for
allylidene, for 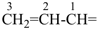 ; and allylidyne, for
; and allylidyne, for 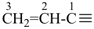 ; are retained for general nomenclature only. Substitution is allowed, but not by alkyl or any other group that extends the carbon chain, or characteristic groups expressed by suffixes. The systematic names ethenyl, ethenylidene, prop-2-en-1-yl, prop-2-en-1-ylidene, and prop-2-en-1-ylidyne, respectively, are the preferred prefix
; are retained for general nomenclature only. Substitution is allowed, but not by alkyl or any other group that extends the carbon chain, or characteristic groups expressed by suffixes. The systematic names ethenyl, ethenylidene, prop-2-en-1-yl, prop-2-en-1-ylidene, and prop-2-en-1-ylidyne, respectively, are the preferred prefix .
The name isopropenyl, for CH2=C(CH3)–, is a retained name but is not used as a preferred prefix . It is acceptable for general use, but substitution is not allowed. The preferred prefix
is prop-1-en-2-yl.
The name styryl, for C6H5-CH=CH–, is a retained name used only in general nomenclature with substitution permitted only on the ring. The preferred prefix is 2-phenylethen-1-yl.
P-32.4 RETAINED NAMES FOR SUBSTITUENT GROUPS DERIVED FROM PARTIALLY SATURATED POLYCYCLIC PARENT HYDRIDES
The prefixes in Table 3.2 are retained but are acceptable only for general nomenclature and may be fully substituted; the preferred prefixes are formed systematically.
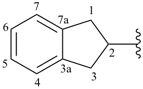
indan-2-yl (also 1-, 4- and 5-isomers)
2,3-dihydro-1H-inden-2-yl (preferred prefix) (also 1-, 4- and 5-isomers)
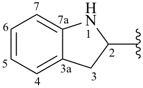
indolin-2-yl (also 1-, 3-, 4-, 5-, 6- and 7-isomers)
2,3-dihydro-1H-indol-2-yl (preferred prefix) (also 1-, 3-, 4-, 5-, 6- and 7-isomers)
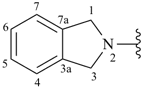
isoindolin-2-yl (also 1-, 4- and 5-isomers)
1,3-dihydro-2H-isoindol-2-yl (preferred prefix) (also 1-, 4- and 5-isomers)
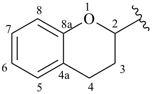
chroman-2-yl (also 3-, 4-, 5-, 6-, 7- and 8-isomers)
3,4-dihydro-2H-chromen-2-yl (also 3-, 4-, 5-, 6-, 7- and 8-isomers)
3,4-dihydro-2H-1-benzopyran-2-yl (also 3-, 4-, 5-, 6-, 7- and 8-isomers; preferred prefixes)
thiochroman-2-yl (S instead of O) (also 3-, 4-, 5-, 6-, 7- and 8-isomers)
3,4-dihydro-2H-thiochromen-2-yl (also 3-, 4-, 5-, 6-, 7- and 8-isomers)
3,4-dihydro-1-benzothiopyran-2-yl (also 3-, 4-, 5-, 6-, 7- and 8-isomers; preferred prefixes)
selenochroman-2-yl (Se instead of O) (also 3-, 4-, 5-, 6-, 7- and 8-isomers)
3,4-dihydro-2H-selenochromen-2-yl (also 3-, 4-, 5-, 6-, 7- and 8-isomers)
3,4-dihydro-2H-1-benzoselenopyran-2-yl (also 3-, 4-, 5-, 6-, 7- and 8-isomers; (preferred prefixes)
tellurochroman-2-yl (Te instead of O) (also 3-, 4-, 5-, 6-, 7- and 8-isomers)
3,4-dihydro-2H-tellurochromen-2-yl (also 3-, 4-, 5-, 6-, 7- and 8-isomers)
3,4-dihydro-2H-1-benzotelluropyran-2-yl (also 3-, 4-, 5-, 6-, 7- and 8-isomers; preferred prefixes)
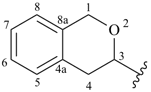
isochroman-3-yl (also 1-, 4-, 5-, 6-, 7- and 8-isomers)
3,4-dihydro-1H-isochromen-3-yl (also 1-, 4-, 5-, 6-, 7- and 8-isomers)
3,4-dihydro-1H-2-benzopyran-3-yl (also 1-, 4-, 5-, 6-, 7- and 8-isomers; preferred prefixes)
isothiochroman-3-yl (S instead of O) (also 1-, 4-, 5-, 6-, 7- and 8-isomers)
3,4-dihydro-1H-isothiochromen-3-yl (also 1-, 4-, 5-, 6-, 7- and 8-isomers)
3,4-dihydro-1H-2-benzothiopyran-3-yl (also 1-, 4-, 5-, 6-, 7- and 8-isomers; preferred prefixes)
isoselenochroman-3-yl (Se instead of O) (also 1-, 4-, 5-, 6-, 7- and 8-isomers)
3,4-dihydro-1H-isoselenochromen-3-yl (also 1-, 4-, 5-, 6-, 7- and 8-isomers)
3,4-dihydro-1H-2-benzoselenopyran-3-yl (also 1-, 4-, 5-, 6-, 7- and 8-isomers; preferred prefixes)
isotellurochroman-3-yl (Te instead of O) (also 1-, 4-, 5-, 6-, 7- and 8-isomers)
3,4-dihydro-1H-isotellurochromen-3-yl (also 1-, 4-, 5-, 6-, 7- and 8-isomers)
3,4-dihydro-1H-2-benzotelluropyran-3-yl (also 1-, 4-, 5-, 6-, 7- and 8-isomers; preferred prefixes)
P-33.0 IntroductionP-33.0 INTRODUCTION
P-33.1 Definitions
P-33.2 Functional suffixes
P-33.3 Cumulative suffixes
This Section includes names of substituents denoting characteristic groups expressed as suffixes. These characteristic groups are essentially those having free valence(s) on atoms such as the chalcogens (O, S, Se, Te) and nitrogen. The concept is extended to carbon atoms linked to halogens, chalcogens and nitrogen, such as –CO-Cl, –CO-OH, –CS-SH, –CHO, –CN. Radicals and ions are expressed by suffixes in substitutive nomenclature although they are not classified as characteristic groups.
P-33.1 DEFINITIONS
Suffixes are divided into ‘functional suffixes’ that express characteristic groups and ‘cumulative suffixes’ that are used to denote radicals and ions. Functional suffixes (P-33.2) are used to denote characteristic groups; they are exclusive because only one can be placed at the end of a name to represent the principal characteristic group or function. Suffixes designating radicals and ions, on the other hand, can be used in association with each other and also in association with functional suffixes (see Chapter P-7). In names, functional suffixes are always attached to the name of the parent hydride, modified or not by ‘ene’ and ‘yne’ endings. ‘Cumulative suffixes’ (see P-33.3) can be attached directly to the names of parent hydrides, modified or not by ‘ene’ and ‘yne’ endings; but when functional suffixes are present, cumulative suffixes are attached to them.
Examples:
| CH4 |  | CH5+ | ||
| methane (PIN) | methanium (PIN) (the suffix ‘-ium’ indicates addition of H+) | |||
| CH4 |  | CH3-NH2 |  | CH3-NH3+ |
| methane (PIN) | methanamine (PIN) (the suffix ‘-amine’ describes substitution) | methanaminium (PIN) (the suffix ‘-ium’ describes addition of H+) | ||
| CH3-CH3 |  | •CH2-CH3 |  | •CH2-CH2• |
| ethane (PIN) | ethyl (PIN; the suffix ‘-yl’ decribes the loss of one hydrogen atom) | ethan-1-yl-2-ylidene (PIN; the the suffix ‘-ylidene’ describes the loss of two hydrogen atoms) |
P-33.2 FUNCTIONAL SUFFIXES
P-33.2.1 Basic functional suffixes
Basic suffixes are those composed only of oxygen and/or nitrogen, with or without association with carbon, as in the case of carboxylic acids, amides, nitriles and aldehydes, and also with sulfur to denote sulfonic acids and sulfinic acids, and the corresponding amides and hydrazides. They are listed in Table 3.3.
The suffix ‘peroxol’, for –OOH, has been added to the list of basic suffixes. It is modified by functional replacement generating the suffixes ‘–OS-thioperoxol’ for –OSH, and ‘–SO-thioperoxol’ for –SOH. The suffix ‘sulfenic acid’, for –SOH, was abandoned in the 1993 Recommendations (ref. 2).
| Formula | Basic suffix | |
| (1) | –CO-OH | carboxylic acid (preferred suffix) |
| (2) | –(C)O-OH | oic acid (preferred suffix) |
| (3) | –SO2-OH | sulfonic acid (preselected suffix) |
| (4) | –SO-OH | sulfinic acid (preselected suffix) |
| (5) | –CO-NH2 | carboxamide (preferred suffix) |
| (6) | –(C)O-NH2 | amide (preferred suffix) |
| (7) | –CO-NHNH2 | carbohydrazide (preferred suffix) |
| (8) | –(C)O-NHNH2 | hydrazide (preferred suffix) |
| (9) | –CN | carbonitrile (preferred suffix) |
| (10) | –(C)N | nitrile (preferred suffix) |
| (11) | –CHO | carbaldehyde (preferred suffix) |
| (12) | –(C)HO | al (preferred suffix) |
| (13) | =O | one (preselected suffix) |
| (14) | –OH | ol (preselected suffix) |
| (15) | –OOH | peroxol (preselected suffix) |
| (16) | –NH2 | amine (preselected suffix) |
| (17) | =NH | imine (preselected suffix) |
P-33.2.2 Derived preferred and preselected suffixes
Derived suffixes are formed in various ways by modifying the basic suffixes of Table 3.3. There is no general rule governing the elision of the final letter ‘o’ of the functional replacement prefixes and infixes before a vowel. It is elided or not according to traditional usage, for example in the suffixes ‘thioic acid’ and ‘imidic acid’. The preferred suffixes are as follows:
(1) Basic suffixes that include a carbon atom are modified by functional replacement by using infixes to indicate the replacement of oxygen atoms by –OO–, –S–, =S, –Se–, =Se, –Te–, =Te, =NH and =NNH2, as indicated in P-15.5; it is to be noted that the letter ‘x’ of ‘carboxylic’ is maintained before a vowel and that the letter ‘o’ is not elided before amide.
Examples:
| –CO-OH | carboxylic acid (preferred suffix) |
| –C(O)-OOH | carboperoxoic acid (preferred suffix) |
| –C(O)-SH | carbothioic S-acid (preferred suffix) |
| –C(Se)-OH | carboselenoic O-acid (preferred suffix) |
| –C(=NH)-OH | carboximidic acid (preferred suffix) |
| –C(=NNH2)-OH | carbohydrazonic acid (preferred suffix) |
| –C(=NH)-SH | carboximidothioic acid (preferred suffix) |
| –CO-NH2 | carboxamide (preferred suffix) |
| –C(Te)-NH2 | carbotelluroamide (preferred suffix) |
| –CO-NHNH2 | carbohydrazide (preferred suffix) |
| –C(S)-NHNH2 | carbothiohydrazide (preferred suffix) |
| –CHO | carbaldehyde (preferred suffix) |
| –CHS | carbothialdehyde (preferred suffix) |
(2) Basic suffixes that include an implied carbon atom are modified by functional replacement, using prefixes to indicate the replacement of oxygen atoms by –OO–, –S–, =S, –Se–, =Se, –Te–, =Te, =NH and =NNH2, as indicated in P-15.5; it is to be noted that the letter ‘o’ is not elided before amide and that an additional ‘o’ is elided from ‘imide’ before ‘oic’ for euphonic reasons.
Examples:
| –(C)O-OH | oic acid (preferred suffix) |
| –(C)O-OOH | peroxoic acid (preferred suffix) |
| –(C)O-SH | thioic S-acid (preferred suffix) |
| –(C)Te-OH | telluroic O-acid (preferred suffix) |
| –(C)(=NH)-OH | imidic acid (preferred suffix) (not imidoic acid) |
| –(C)(=NNH2)-OH | hydrazonic acid (preferred suffix) |
| –(C)(=NH)-SeH | imidoselenoic acid (preferred suffix) |
| –(C)O-NH2 | amide (preferred suffix) |
| –(C)S-NH2 | thioamide (preferred suffix) |
| –(C)O-NHNH2 | hydrazide (preferred suffix) |
| –(C)S-NHNH2 | thiohydrazide (preferred suffix) |
| –(C)HO | al (preferred suffix) |
| –(C)HSe | selenal (preferred suffix) |
(3) Basic suffixes that do not include a carbon atom are modified by functional replacement nomenclature using prefixes to indicate the replacement of oxygen atoms by other chalcogen atoms.
Examples:
| =O | one (preselected suffix) |
| =S | thione (preselected suffix) |
| =Se | selone (preselected suffix) (not selenone) |
| =Te | tellone (preselected suffix) (not tellurone) |
| –OH | ol (preselected suffix) |
| –SH | thiol (preselected suffix) |
| –OOH | peroxol (preselected suffix) |
| –OSH | OS-thioperoxol (preselected suffix) |
(4) The stem ‘sulf’ is replaced by ‘selen’ and ‘tellur’ to generate the selenium and tellurium analogues of sulfonic and sulfinic acids.
Examples:
| –SO2-OH | sulfonic acid (preselected suffix) |
| –SO-OH | sulfinic acid (preselected suffix) |
| –SeO2-OH | selenonic acid (preselected suffix) |
| –TeO-OH | tellurinic acid (preselected suffix) |
(5) Suffixes of the type ‘sulfonic acid’ and analogues are modified by functional replacement by using infixes to indicate the replacement of oxygen atoms by –OO–, –S–, =S, –Se–, =Se, –Te–, =Te, =NH and =NNH2, as indicated in P-15.5.
Examples:
| –SO2-OH | sulfonic acid (preselected suffix) |
| –SO2-OOH | sulfonoperoxoic acid (preselected suffix) |
| –S(=NNH)2-OH | sulfonodihydrazonic acid (preselected suffix) |
| –SeO-OH | seleninic acid (preselected suffix) |
| –SeO-SH | seleninothioic S-acid (preselected suffix) |
| –TeO2-OH | telluronic acid (preselected suffix) |
| –Te(O)(=NH)-OH | telluronimidic acid (preselected suffix) |
| –SO-OH | sulfinic acid (preselected suffix) |
| –S(=NNH2)-OH | sulfinohydrazonic acid (preselected suffix) |
(6) Names of amides and hydrazides are formed by replacing the ‘ic acid’ ending in suffixes by ‘amide’ or ‘hydrazide’, respectively ; a euphonic letter ‘o’ is added as required:
Examples:
| –(C)(=NH)-OH | imidic acid (preferred suffix) |
| –(C)(=NH)-NH2 | imidamide (preferred suffix) |
| –C(=NH)-OH | carboximidic acid (preferred suffix) |
| –C(=NH)-NH2 | carboximidamide (preferred suffix) |
| –(C)(=NNH2)-OH | hydrazonic acid (preferred suffix) |
| –(C)(=NHNH2)-NHNH2 | hydrazonohydrazide (preferred suffix) |
| –SO2-OH | sulfonic acid (preselected suffix) |
| –SO2-NH2 | sulfonamide (preselected suffix) |
| –SeO-OH | seleninic acid (preselected suffix) |
| –SeO-NHNH2 | seleninohydrazide (preselected suffix) |
(7) Suffixes with –NH2 and =NH groups substituted by an –OH group are named in two ways:
(1) are named as N-hydroxy derivatives of amides or imidic acids;(2) by modifying the ‘-oic acid’ or ‘-carboxylic acid’ suffix of a systematically named acid to ‘-hydroxamic acid’ or ‘-carbohydroxamic acid’ and ‘-hydroximic acid’ or ‘-carbohydroximic acid’, or the ‘-ic acid’ ending of the retained name of an acid to ‘-hydroxamic acid’ or ‘-hydroximic acid’. The letter ‘o’ is added for euphony between ‘h’ and a preceding consonant.
Method (1) is used for preferred IUPAC names.
Examples:
CH3-CH2-CO-NH-OH
N-hydroxypropanamide (PIN)
propanohydroxamic acid
CH3-CH2-C(=NH)-OH
propanimidic acid (PIN)
CH3-CH2-C(=N-OH)-OH
N-hydroxypropanimidic acid (PIN)
propanohydroximic acid
Suffixes used to denote radical and ionic centers in a parent structure are given in Table 3.4. They are classified in decreasing order of seniority, radicals > anions > cations. Suffixes are added to the name of a parent hydride in the customary manner, or to suffixes expressing another type of radical or ion, or to suffixes denoting characteristic groups. Names of radicals are formed in the same manner as substituent groups (see P-29.2), with the exception that di- and trivalent radicals centered on a single atom are denoted by the suffixes ‘ylidene’ and ‘ylidyne’, respectively, and never by ‘diyl’ or ‘triyl’.
Note: ‘ene’, ‘yne’, etc. are considered endings not suffixes; they are cumulative endings.
| Operation | Suffix | Ending | |
| Radicals | Loss of H• | -yl | |
| Loss of 2 H• | |||
| from one atom | -ylidene | ||
| from different atoms | -diyl | ||
| Loss of 3 H• | |||
| from one atom | -ylidyne | ||
| from different atoms | -triyl | ||
| -ylylidene | |||
| etc. | |||
| Anions | Loss of H+ | ||
| Addition of H– | -uide | ate, ite | |
| Cations | Loss of H– | -ylium | |
| Addition of H+ | -ium | ||
Examples:
CH3-CH2•
ethyl (PIN)
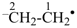
ethan-2-id-1-yl (PIN)
CH3-NH2
methanamine (PIN)
CH3-NH3+
methanaminium (PIN)
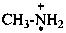
methanaminiumyl (PIN)
CH3-NH•
methanaminyl (PIN)
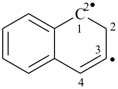
naphthalen-3-yl-1(2H)-ylidene (PIN)
P-34.0 IntroductionP-34.0 INTRODUCTION
P-34.1 Retained functional parent compounds
P-34.2 Substituent groups related to functional parent compounds
Many trivial and semisystematic names have been used in organic chemistry. As systematic names have been increasingly recommended, the number of retained names (trivial and semisystematic) has been gradually reduced, in the 1979 Rules (ref. 1) and again in the 1993 Guide (ref. 2). Functional parent compounds have been defined and discussed in Section P-15.1.2 dealing with substitutive nomenclature. This subsection describes the 2005 codification of the list of functional parent compounds established in 1993 as far as their classification as preferred IUPAC names or names that are to be used only in general and specialized (see Chapter P-10) nomenclature is concerned.
Names recommended as preferred IUPAC names are described in the following section, P-34.1. Names that are recommended for general and specialized nomenclature are discussed in P-34.2, Chapter P-6, and Chapter P-10 along with systematic substitutive names recommended for the different classes of compounds.
P-34.1 RETAINED FUNCTIONAL PARENT COMPOUNDS
The retained names of the following functional parent compounds are used as preferred IUPAC names, as well as in general and specialized nomenclature. The lists in P-34.1.1 and P-34.1.2 are exhaustive for preferred IUPAC names; for more retained names of functional parent compounds recommended for use in general and specialized nomenclature, see P-34.1.3. The type of substitution (allowed or not) is specified for each compound in accordance with the general methodology indicated in P-15.1.8.
Inorganic functional parent compounds are acids and related compounds denoted by retained names that are preselected (see P-12.2); they are discussed in P-34.1.4.
P-34.1.1 Organic functional parent compounds (arranged by characteristic group class)P-34.1.1 Organic functional parent compounds (arranged by characteristic group class)
P-34.1.2 Organic functional parent compounds (arranged alphabetically)
P-34.1.3 Organic functional parent compounds for general and specialized nomenclature
P-34.1.4 Inorganic functional parent compounds
P-34.1.1.1 Acids
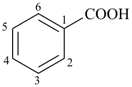
benzoic acid (PIN)
(substitution allowed; see P-65.1.1.1)
benzenecarboxylic acid
H2N-COOH
carbamic acid (PIN)
(substitution allowed; see P-65.2.1.1)
carbonamidic acid
HO-CO-OH
carbonic acid (PIN) (see P-65.2.1)
NC-OH
cyanic acid (PIN)
carbononitridic acid (see P-65.2.2)
H-COOH
formic acid (PIN)
(limited substitution see P-65.1.8)
methanoic acid
HOOC-COOH
oxalic acid (PIN)
(see P-65.1.1.1)
ethanedioic acid
H2N-CO-COOH
oxamic acid (PIN)
(substitution allowed; see P-65.1.6.1)
amino(oxo)acetic acid
H2N-C(=NH)-OH
carbamimidic acid
(PIN; see P-65.2.1.3)
carbonamidimidic acid

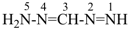
formazan (PIN)
(see P-68.3.1.3.5)
(diazenylmethylidene)hydrazine
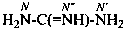
guanidine (PIN)
(substitution allowed; see P-66.4.1.2.1)
carbonimidic diamide
H2N-OH
hydroxylamine
(preselected name) (see P-68.3.1.1.1)
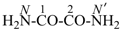
oxamide (PIN)
(substitution allowed; see P-66.1.1.1.2.1)
oxalic diamide
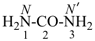
urea (PIN)
(substitution allowed; see P-66.1.6.1.1)
carbonic diamide
| Retained name (PIN) or preselected name | Alternative names |
| acetic acid (substitution allowed; see P-65.1.1.1) | ethanoic acid |
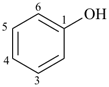
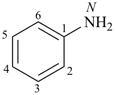
| aniline (substitution allowed; see P-62.2.1.1.1) | benzenamine |
| anisole (no substitution on anisole for PINs; for general nomenclature substitution is permitted on the ring and on the α-methoxy group only by groups listed in P-15.1.8.2; see also P-63.2.3) | methoxybenzene |
| benzoic acid (substitution allowed; see P-65.1.1.1) | benzenecarboxylic acid |
| carbamic acid (substitution allowed; see P-65.2.1.1) | carbonimidic diamide |
| carbamimidic acid (substitution allowed; see P-65.2.1.3) | |
| carbonic acid (see P-65.2) | |
| cyanic acid (see P-65.2.2) | carbononitridic acid |
| formazan (substitution allowed; see (hydrazinylidenemethyl)diazene P-68.3.1.3.5) | (diazenylmethylidene)hydrazine |
| formic acid (limited substitution allowed; see P-65.1.8) | methanoic acid |
| guanidine (substitution allowed; see P-66.4.1.2.1) | carbonimidic diamide |
| hydroxylamine (special substitution; see P-68.3.1.1.1) | |
| oxamide (substitution allowed; see P-66.1.1.1.2) | oxalic diamide |
| oxamic acid (substitution allowed; see P-65.1.6.1) | |
| oxalic acid (see P-65.1.1.1) | ethanedioic acid |
| phenol (substitution allowed; see P-63.1.1.1) | benzenol |
| urea (substitution allowed; see P-66.1.6.1.1) | carbonic diamide |
P-34.1.3 Organic functional parent compounds for general and specialized nomenclature
Functional parent compounds that were recommended in the 1979 recommendations (ref. 1) and /or the 1993 Guide (ref. 2) past can be used in general organic nomenclature. They are also used in biochemical nomenclature, the nomenclature of polymers, and in the nomenclature of natural products. Their formulae and names are described in Chapters P-6 and P-10. The different classes are: hydroxy compounds and ethers (see P-63), carbonyl compounds (see P-64), carboxylic acids (see P-65), amines (see P-62), sulfur compounds (see P-66.1.1.4.2) and sulfamic acid (see P-67.1.2.4.1.1), acyclic polynitrogen compounds (see P-66.1.6, P-68.3.1.3), and halogen compounds (see P-68.5).
Structures of alkaloids, steroids, terpenes, and similar compounds are given in Appendix 3.
P-34.1.4 Inorganic functional parent compounds
These compounds are described in P-67.1.1 and P-67.2.1.
P-34.2 SUBSTITUENT GROUPS RELATED TO FUNCTIONAL PARENT COMPOUNDS
P-34.2.1 Organic substituent groups (arranged by classes)
P-34.2.1.1 Acyl groups
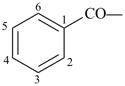
benzoyl (preferred prefix)
(full substitution, see P-65.1.7.2.1 )
benzenecarbonyl
H2N-C(=NH)–
carbamimidoyl (preferred prefix)
(full substitution, see P-65.2.1.5 )
C-aminocarbonimidoyl
H2N-CO–
carbamoyl (preferred prefix)
(full substitution, see P-65.2.1.5 )
aminocarbonyl
–CO–
carbonyl (preferred prefix)
(see P-65.2.1.5 )
H2N-CO-CO–
oxamoyl (preferred prefix)
(full substitution; see P-66.1.1.4.1.2 )
H-CO–
formyl (preferred prefix)
(limited substitution; see P-65.1.7.2.1 )
oxomethyl
–CO-CO–
oxalyl (preferred prefix)
(see P-65.1.7.2.1)
ethanedioyl
dioxoethanediyl
P-34.2.1.2 Substituent groups derived from hydroxy compounds
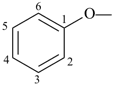
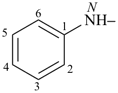
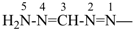
formazan-1-yl (preferred prefix)
(full substitution; see P-68.3.1.3.5.2)
(hydrazinylidenemethyl)diazenyl
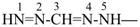
formazan-5-yl (preferred prefix)
(full substitution; see P-68.3.1.3.5.2)
(diazenylmethylidene)hydrazinyl
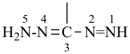
formazan-3-yl (preferred prefix)
(full substitution; see P-68.3.1.3.5.2)
diazenyl(hydrazinylidene)methyl
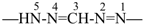
formazan-1,5-diyl (preferred prefix)
(full substitution; see P-68.3.1.3.5.2)
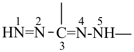
formazan-3,5-diyl (preferred prefix)
(full substitution; see P-68.3.1.3.5.2)
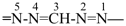
formazan-1-yl-5-ylidene (preferred prefix)
(full substitution; see P-68.3.1.3.5.2)
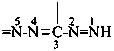
formazan-3-yl-5-ylidene (preferred prefix)
(full substitution; see P-68.3.1.3.5.2)
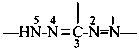
formazan-1,3,5-triyl (preferred prefix)
(full substitution; see P-68.3.1.3.5.2)
H2N-C(=NH)-NH—
carbamimidoylamino (preferred prefix)
(see P-66.4.1.2.1.3)
guanidino
(H2N)2C=N—
(diaminomethylidene)amino (preferred prefix)
(see P-66.4.1.2.1.3)
H2N-CO-CO-NH—
oxamoylamino (preferred prefix)
(see P-66.1.1.4.5.1)
—HN-CO-NH—
oxalylbis(azanediyl) (preferred prefix)
(see P-66.1.1.4.5.2)
H2N-CO-NH—
carbamoylamino (preferred prefix)
(see P-66.1.6.1.1.3)
(not ureido)
—HN-CO-NH—
carbonylbis(azanediyl) (preferred prefix)
(see P-66.1.6.1.1.3)
| Preferred Prefxes | Alternative names |
| acetyl (substitution allowed except that the carbon chain cannot be extended; see P-65.1.7.2.1) | ethanoyl |
| anilino (full substitution allowed; see P-62.2.1.1.1) | phenylamino |
| benzoyl (substitution allowed; see P-65.1.7.2.1) | benzenecarbonyl phenylcarbonyl |
| carbamimidoyl (substitution allowed; P-65.2.1.5) | C-aminocarbonimidoyl |
| carbamimidoylamino (see P-66.4.1.2.1.3) | |
| carbamoyl (substitution allowed; see P-65.2.1.5) | aminocarbonyl |
| carbamoylamino (see P-66.1.6.1.1.3) | |
| carbonyl (see P-65.2.1.5) | |
| carbonylbis(azanediyl) (see P-66.1.6.1.1.3) | |
| (diaminomethylidene)amino (see P-66.4.1.2.1.3) | |
| formazan-1,5-diyl (substitution allowed; P-68.3.1.3.5.2) | |
| formazan-3,5-diyl (substitution allowed; P-68.3.1.3.5.2) | |
| formazan-1,3,5-triyl (substitution allowed; see P-68.3.1.3.5.2) | |
| formazan-1-yl (substitution allowed; see P-68.3.1.3.5.2) | (hydrazinylidenemethyl)diazenyl |
| formazan-3-yl (substitution allowed; see P-68.3.1.3.5.2) | diazenyl(hydrazinylidene)methyl |
| formazan-5-yl (substitution allowed; see P-68.3.1.3.5.2) | (diazenylmethylidene)hydrazinyl |
| formazan-1-yl-5-ylidene (substitution allowed; see P-68.3.1.3.5.2) | |
| formazan-3-yl-5-ylidene (substitution allowed; see P-68.3.1.3.5.2) | |
| formyl (limited substitution allowed; see P-65.1.7.2.1) | methanoyl |
| oxamoylamino (see P-66.1.1.4.5.1) | |
| oxalylbis(azanediyl) (see P-66.1.1.4.5.2) | |
| oxamoyl (substitution allowed; see P-66.1.1.4.1.2) | |
| oxalyl (see P-65.1.7.2.1) | ethanedioyl |
| phenoxy (substitution allowed; see P-63.2.2.2) | phenyloxy |
P-34.2.3 Substituent group names derived from organic compounds used in general and specialized nomenclature
These are discussed in Chapters P-6 and P-10.
P-34.2.4 Preselected substituent group names
Examples:
>N-OH
hydroxyazanediyl (preselected prefix)
(see P-68.3.1.1.1.5)
–O-NH2
aminooxy (preselected prefix)
(see P-68.3.1.1.1.5)
P-35.0 IntroductionP-35.0 INTRODUCTION
P-35.1 General methodology
P-35.2 Simple prefixes denoting characteristic groups
P-35.3 Compound substituent prefixes
P-35.4 Complex substituent prefixes
P-35.5 Mixed substituent prefixes
Prefixes used to designate characteristic groups in substitutive nomenclature are those having free valence(s) attached to an atom of Group 17 (F, Cl, Br, and I) or Group 16 (O, S, Se, and Te), or to nitrogen. They have retained names or they are formed systematically by the methods described for substituent groups derived from parent hydrides (see P-29). Oxygen and nitrogen atoms can also be attached to a carbon atom, for example –CO-OH, –CO-NH2, and –CO-CH2-CH3, or to a chalcogen atom, for example –S(O)2-OH, –Se(O)2-OH. These prefixes correspond to suffixes listed in P-33, for example the prefix ‘hydroxy’ for –OH corresponds to the suffix ‘ol’ for the same group. Prefixes are also derived from functional parents as defined in Sections P-34 and P-15.1.2, in particular acyl groups such as ‘acetyl’, for –CO-CH3, derived from acetic acid, and phosphoryl, for –PO<.
Prefixes may have both a retained name and a systematic name only one of which can be a preferred IUPAC name. In order to facilitate the selection of preferred IUPAC names, clear indications are given in Chapter P-6 for each class of compounds. The prefixes are also alphabetically listed in Appendix 2, with indications concerning their status as preferred IUPAC names as well as their use in substitutive nomenclature and as functional replacement prefixes.
In this Section, the different types of prefixes used in substitutive nomenclature and in multiplicative nomenclature are described.
P-35.1 GENERAL METHODOLOGY
Substitutive prefixes corresponding to characteristic groups and functional parent compounds may be classified as simple (see P-29.1.1), compound (see P-29.1.2), and complex (see P-29.1.3); mixed substituent groups are complex substituent groups formed by the combination of substitutive and additive operations.
The multiple occurrence of simple prefixes is denoted by the basic numerical terms ‘di’, ‘tri’, etc., or by the derived prefixes ‘bis’, ‘tris’, ‘tetrakis’, etc., in order to distinguish between two simple prefixes and those including a basic multiplying term, for example disulfanyl, ‘–SSH’, and bis(sulfanyl), two –SH groups. Compound and mixed substituent prefixes require the derived multiplying terms ‘bis’, ‘tris’, ‘tetrakis’, etc., to designate their multiplicity in substitutive nomenclature.
Simple prefixes are either retained or systematically formed as follows:
(1) by subtracting hydrogen atom(s) from parent hydrides (described as substituent groups derived from parent hydrides in Section P-29), for example ‘sulfanyl’, –SH, and ‘diselanyl’, –SeSeH; from functional parent compounds, for example acetonyl, CH3-CO-CH2–; or from a contracted name, for example ‘methoxy’, CH3-O–;
(2) acyl groups are formed by subtracting all −OH groups from oxoacids for example ‘acetyl’, CH3-CO–, and ‘carbonyl’, >C=O.
P-35.2 SIMPLE PREFIXES DENOTING CHARACTERISTIC GROUPS
P-35.2.1 Retained traditional prefixes
–Cl
chloro (preselected prefix)
–Br
bromo (preselected prefix)
–I
iodo (preselected prefix)
–OH
hydroxy (preselected prefix)
=O
oxo (preselected prefix)
–O–
oxy (preselected prefix)
–COOH
carboxy (preferred prefix)
–SO2-OH
sulfo (preselected prefix)
selenono (Se instead of S; preselected prefix)
tellurono (Te instead of S; preselected prefix)
–SO-OH
sulfino (preselected prefix)
selenino (Se instead of S; preselected prefix)
tellurino (Te instead of S; preselected prefix)
–NH2
amino (preselected prefix)
–N3
azido (preselected prefix)
=NH
imino (to the same atom; preselected prefix)
–N<
nitrilo (to different atoms; preselected prefix)
Note: In order to distinguish between HN= and –HN– (both are commonly called ‘imino’), and between –N< and –N= (both are commonly called ‘nitrilo’), systematic names based on the parent hydride azane are recommended for the latter in each pair.
=N2
diazo (preselected prefix)
–NC
isocyano (preferred prefix)
–CN
cyano (preferred prefix )
–NCO
isocyanato (preferred prefix)
isothiocyanato (S instead of O; preferred prefix)
isoselenocyanato (Se instead of O; preferred prefix)
isotellurocyanato (Te instead of O; preferred prefix)
Systematic names are formed by the general methodology described in P-29.3.1.
Examples:
–S–
sulfanediyl (preselected prefix)
thio
=S
sulfanylidene (preselected prefix)
thioxo
–SS–
disulfanediyl (preselected prefix)
dithio
–SeH
selanyl (preselected prefix)
(not selenyl)
–Se–
selanediyl (preselected prefix)
seleno
=Se
selanylidene (preselected prefix)
selenoxo
–SeSe–
diselanediyl (preselected prefix)
diseleno
–TeH
tellanyl (preselected prefix)
–Te–
tellanediyl (preselected prefix)
telluro
=Te
tellanylidene (preselected prefix)
telluroxo
–TeTe–
ditellanediyl (preselected prefix)
ditelluro
H2N-NH–
hydrazinyl (preslected prefix)
diazanyl
HN=N–
diazenyl (preselected prefix)
–NH–
azanediyl (preselected prefix) (to different atoms)
The systematic name based on the parent hydride azane is the preferred IUPAC name for –HN– in order to distinguish between HN= and –HN– both of which are commonly called ‘imino’
≡N
azanylidyne (preselected prefix) (to the same atom)
The systematic name based on the parent hydride azane is the preferred IUPAC names for –N= in order to distinguish between –N< and –N= both of which are commonly called ‘nitrilo’.
P-35.2.3 Simple prefixes derived from functional parent compounds
A few simple prefixes are derived from functional parent compounds described in P-34 and in P-67.
Examples:
–PO<
phosphoryl (preselected prefix; see P-67.1.4.1.1.2)
–SO2–
sulfonyl (preselected prefix; see P-65.3.2.3)
sulfuryl
–SO–
sulfinyl (preselected prefix; see P-65.3.2.3)
thionyl
–SeO2–
selenonyl (preselected prefix; see P-65.3.2.3)
–SeO–
seleninyl (preselected prefix; see P-65.3.2.3)
–TeO2–
telluronyl (preselected prefix; see P-65.3.2.3)
–TeO–
tellurinyl (preselected prefix; see P-65.3.2.3)
CH3-CO–
acetyl (preferred prefix; see P-65.1.7.2.1)
C6H5-CO–
benzoyl (preferred prefix; see P-65.1.7.2.1)
P-35.3.1 Names of compound prefixes derived from suffixes or functional parent compounds may be formed by substituting simple prefixes into other simple prefixes. When there is a choice, P-57.4 provides for the selection of a preferred compound prefix.
Examples:
| –SSeH | selanylsulfanyl (preselected prefix) |
| –NH-Cl | chloroamino (preselected prefix) |
| –NH-CH3 | methylamino (preferred prefix) |
| –PH-Cl | chlorophosphanyl (preselected prefix) |
P-35.3.2 Names of compound prefixes derived from suffixes or functional parent compounds may be formed by the additive operation called concatenation. This is used to assemble simple mono-, di-, tri-, and tetravalent prefixes. Hydrocarbyl divalent substituents can be attached to prefixes expressing characteristic groups. This method is used when no hydrogen atoms are present for substitution (see P-15.1); it is also used to form substituent groups in multiplicative nomenclature (see P-15.3.1.2.2)
Examples:
| –O-CH2-CH2-CH2-CH2-CH3 | pentyloxy (preferred prefix) |
| –O-CH2-C6H5 | benzyloxy (preferred prefix) |
| –CO-Cl | carbonochloridoyl (preferred prefix) chlorocarbonyl |
| –C(=NH)-OH | C-hydroxycarbonimidoyl (preferred prefix) hydroxy(imino)methyl |
| –C(=N-NH2)-OH | C-hydroxycarbonohydrazonoyl (preferred prefix) hydrazinylidene(hydroxy)methyl |
| –O-CH2-CH2-O– | ethane-1,2-diylbis(oxy) (preferred prefix) |
| >N-CH2-N< | methylenedinitrilo (preferred prefix) |
| >P(S)-CH2-P(S)< | methylenebis(phosphorothioyl) (preferred prefix) |
P-35.4 COMPLEX SUBSTITUENT PREFIXES
P-35.4.1 Names of complex substituent prefixes may be formed by substituting a simple or compound substituent prefix into a compound substituent prefix. When there is a choice, P-57.4 provides for the selection of a preferred complex substituent prefix.
Examples:
| –NH-S-SeH | (selanylsulfanyl)amino (preselected prefix) |
| –NH-CH2Cl | (chloromethyl)amino (preferred prefix) |
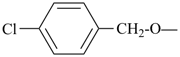 | (4-chlorophenyl)methoxy (preferred prefix) |
| –PH-NH-OCH3 | (methoxyamino)phosphanyl (preferred prefix) |
P-35.4.2 Names of complex substituent prefixes may be formed by adding simple or compound substituent prefixes to a compound substituent prefix by the process called ‘concatenation’.
Examples:
| –CO-O-CH2-C6H5 | (benzyloxy)carbonyl (preferred prefix) |
| –O-CS-OCH3 | (methoxycarbonothioyl)oxy (preferred prefix) |
| –CS-O-P(O)(OCH3)2 | [(dimethoxyphosphoryl)oxy]carbonothioyl (preferred prefix) |
P-35.5 MIXED SUBSTITUENT PREFIXES
P-35.5.1 Mixed substituent prefix names are formed by combining the substitutive and additive operations.
Examples:
| CH3-CH2-O-SO-NH– | (ethoxysulfinyl)amino (preferred prefix) |
| CH3-CO-S-CO– | (acetylsulfanyl)carbonyl (preferred prefix) |
| CH3-CO-O-NH-SO-O– | {[(acetyloxy)amino]sulfinyl}oxy (preferred prefix) |
| (HS)2(O)P-NH– | [bis(sulfanyl)phosphoryl]amino (preselected prefix) |
Return to:
Blue Book home page.