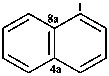
naphthalene (PIN)
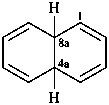
4a,8a-dihydronaphthalene (PIN)
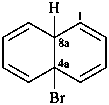
4a-bromo-4a,8a-dihydronaphthalene (PIN)
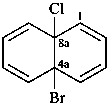
4a-bromo-8a-chloro-4a,8a-dihydronaphthalene (PIN)
Division VIII Chemical Nomenclature and Structure Representation Division
(P-60 to P-65)
(continued with P-66 to P-69)
P-60 Introduction
P-61 Substitutive nomenclature: prefix mode
P-62 Amines and imines
P-63 Hydroxy compounds, ethers, peroxols, peroxides and chalcogen analogues
P-64 Ketones, pseudoketones and heterones, and chalcogen analogues
P-65 Acids, acyl halides and pseudohalides, salts, esters, and anhydrides
P-66 Amides, imides, hydrazides, nitriles, and aldehydes
P-67 Mononuclear and polynuclear noncarbon acids and their functional replacement analogues as functional parents for naming organic compounds
P-68 Nomenclature for organic compounds of the group 13, 14, 15, 16, and 17 elements not included in sections P-62 through P-67
P-69 Nomenclature for organometallic compounds
The recommendations in this Chapter illustrate how the general principles and specific rules set out in the preceding sections are applied to various types of compounds.
P-60.1 TOPICAL OUTLINE
Section P-61 describes hydrocarbons that are named substitutively only by the prefix mode. It includes and exemplifies compounds formed by substituting parent hydrides by substituents derived from other parent hydrides and by characteristic groups that are always used as prefixes when applied to hydrocarbons.
Sections P-62 to P-66 include compounds that are named, in substitutive nomenclature, by suffixes and prefixes, and by means of other types of nomenclature. The traditional classes from acids to imines are described (see P-41).
Section P-67 describes nomenclature of organic derivatives of noncarbon acids and their functional replacement analogues.
Section P-68 covers the nomenclature of organic compounds of the Groups 13, 14, 15, 16, and 17 elements not included in Sections P-62 to P-67.
Section P-69 describes nomenclature for organometallic compounds.
P-60.2 PRESENTATION OF NAMES.
Names described in this Chapter are presented in a systematic way. General methods recommended to generate IUPAC preferred names are all described in a simplified way with reference to the following full descriptions:
(a) names formed substitutively using suffixes follow the general method described in P-15.1. Substitutive names are formed by adding a suffix such as ‘al’ ‘ol’, ‘yl’, ‘carbaldehyde’, ‘carboxylic acid’, etc., to the name of a parent hydride, with elision of the final letter ‘e’ of the parent hydride, if any, before ‘a’, ‘i’, ‘o’, ‘u’, and ‘y’;(b) names formed substitutively by using prefixes follow the general method. Substitutive names are formed by adding a prefix such as amino, hydroxy, etc., to the name of the parent hydride or parent compound; in order to preserve their formal identity, there is no elision of the last letter of these prefixes;
(c) names formed by functional class nomenclature follow the general method described in P-15.2. Functional class names are formed by citing the name of the class, such as alcohol, oxide, ketone, etc., preceded by the name of the substituent groups cited in alphabetical order and separated by a space, if required;
(d) names formed by skeletal replacement (‘a’) nomenclature follow the methodology described in P-15.4;
(e) functional parents are discussed in terms of preferred names and names that can be used in general nomenclature.
The method to generate preferred IUPAC names is indicated by a phrase such as ‘This method generates preferred IUPAC names’ or ‘Method (1) leads to preferred IUPAC names’. The abbreviation ‘PIN’ is placed after names that are ‘preferred IUPAC names’. Names that were recommended in the past but are not included in these recommendations are described parenthetically by the phrase ‘no longer recommended’. For example, the prefix ‘methylene’ is ‘no longer recommended’ in IUPAC nomenclature to designate the =CH2 group.
Names preceded by ‘not’ are names that are not constructed in accordance with the rule as described in this Chapter. Thus, they are ‘incorrect names’. As they are not alternatives to preferred IUPAC names, they must not be used. For example, the name ‘ethanolamine’, which is still widely used, is badly constructed because of the presence of two suffixes; it is not an alternative to the preferred IUPAC name, ‘2-aminoethan-1-ol’.
P-61 SUBSTITUTIVE NOMENCLATURE: PREFIX MODE
P-61.0 Introduction
P-61.1 General methodology
P-61.2 Hydrocarbyl groups and corresponding di- and polyvalent groups
P-61.3 Halogen compounds
P-61.4 Diazo compounds
P-61.5 Nitro and nitroso compounds
P-61.6 Heterones
P-61.7 Azides
P-61.8 Isocyanates
P-61.9 Isocyanides
P-61.10 Fulminates and isofulminates
P-61.11 Polyfunctional compounds
P-61.0 INTRODUCTION
This subsection describes the names of compounds formed by substitutive nomenclature that includes only prefixes denoting substituent groups and/or characteristic groups. These prefixes are detachable and cited in a name in alphanumerical order.
Hydrocarbyl groups and their corresponding polyvalent groups (substituent groups derived from hydrocarbon parent hydrides) are included in this subsection because they occupy the penultimate rank in the seniority order of classes (see P-41) and thus are treated as prefixes in presence of a higher class. A similar situation prevails for halogen compounds in their standard bonding number, which are at the bottom in the order of seniority of classes (see P-41).
Diazo compounds, nitro and nitroso compounds, azides, isocyanates, isocyanides, and fulminates/isofulminates are also included in this Section. Ethers, peroxides, and acetals are not considered in this Section, but are treated at length in association with hydroxy compounds and aldehydes (see P-63.2, P-63.3, and P-66.6.5, respectively).
The characteristic groups described here (see Table 5.1) are referred to as ‘characteristic groups denoted, in substitutive nomenclature, only as prefixes’ (see R-4.1, ref. 2). This statement must not be interpreted as a must for always using these characteristic groups as prefixes. Substitutive nomenclature is based on a seniority system based on classes. The senior class must be determined first (see P-41).
P-61.1 GENERAL METHODOLOGY
Substitutive nomenclature is based on the substitutive operation involving the exchange of one or more hydrogen atoms of a parent hydride or parent compound for another atom or group. This process is expressed by either a prefix or suffix denoting the atom or group being introduced. Substitution is not possible when no hydrogen atoms are present. However, if hydrogen atoms are added to a structure by an additive operation (to a double bond, for example), substitution then becomes possible. The formal addition of hydrogen atoms must precede the substitution operation when atoms or groups denoted by prefixes are involved; thus, they are cited after the alphabetized prefixes.
This is a change from previous recommendations. In these recommendations the prefix ‘hydro’ is detachable but not alphabetized with other substituent prefixes. In names, it is cited immediately before the name of the parent hydride, after alphabetized prefixes and before nondetachable prefixes.
Examples:


4a,8a-dihydronaphthalene (PIN)

4a-bromo-4a,8a-dihydronaphthalene (PIN)

4a-bromo-8a-chloro-4a,8a-dihydronaphthalene (PIN)
The seniority order of parent structures, the principal chain, and the senior ring system are chosen in accordance with Rule P-44.
When there is a choice for numbering, the general rule described in P-14.4 is applied. The starting point and the direction of numbering of a compound are chosen so as to give lowest locants to the following structural features (if present) considered successively in the order given until a decision is reached:
(a) fixed numbering, as for naphthalene, bicyclo[2.2.2]octane, etc.;(b) heteroatoms in heterocycles and in acyclic parent structures;
This is a change for acyclic parent structures. Heteroatoms in chains are now considered as part of the parent hydride and, as such, have seniority over suffixes for numbering. (c) indicated hydrogen [for unsubstituted compounds; a higher locant may be needed at another position to provide for a substituent suffix in accordance with structural feature (d)];
(d) principal group named as suffix;
(e) added indicated hydrogen (consistent with the structure of the compound and in accordance with further substitution);
(f) saturation/unsaturation (‘hydro’/‘dehydro’ prefixes) or unsaturation (‘ene’/‘yne’ endings);
In acyclic parent structures the order of seniority between unsaturation and length of chain given in earlier recommendations is reversed. Thus, the first criterion to be considered in choosing a preferred parent acyclic chain is the length of the chain; unsaturation is now the second criterion. (g) substituents named as prefixes (low locants are allocated for substituents regardless of kind; then, if necessary, in the order of citation in the name).
P-61.2 HYDROCARBYL GROUPS AND CORRESPONDING DI- AND POLYVALENT GROUPS
Only substituted hydrocarbons are discussed here. For substitution on other parent hydrides see P-68.1 for Group 13, P-68.2 for Group 14, P-68.3 for Group 15, P-68.4 for Group 16, and P-68.5 for Group 17.
Substituted hydrocarbons for which a parent hydride name is not available (see Chapter P-2) have a name that consists of a parent hydride name and appropriate substitutive prefixes derived from other parent hydrides.
P-61.2.1 Acyclic hydrocarbons
Names of substituted acyclic hydrocarbons are formed substitutively by selecting the principal chain in accordance with rule P-44. This rule has been modified from previous rules; seniority is now given to the length of the chain rather than to unsaturation (see P-44.3).
In a change from previous recomendations, the order of seniority between unsaturation and length of chain given in earlier recommendations is reversed. Thus, the first criterion to be considered in choosing a preferred parent acyclic chain is the length of the chain; unsaturation is now the second criterion.
The name ‘isoprene’ is retained, but no substitution is allowed. (see P-31.1.2.1). The names ‘isobutane’, ‘isopentane’ and ‘neopentane’ are no longer recommended.
Examples:
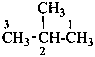
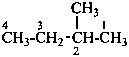
2-methylbutane (PIN)
(not isopentane)
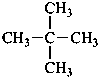
2,2-dimethylpropane (PIN)
(not neopentane)
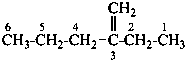
3-methylidenehexane (PIN)
(not 2-ethylpent-1-ene; the longer chain now supersedes
a shorter unsaturated chain; see P-44.3)
Names of rings or cyclic systems substituted by rings or ring systems are formed in accordance with the seniority order of rings and ring systems (see P-44.2.1 and P-44.4.1).
Examples:
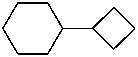
cyclobutylcyclohexane (PIN)
(cyclohexane has more ring atoms than cyclobutane; see P-44.2.1)
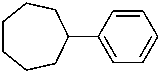
phenylcycloheptane (PIN)
(cycloheptane has more ring atoms than benzene; see P-44.2.1)
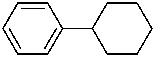
cyclohexylbenzene (PIN)
(benzene has more multiple bonds than cyclohexane; see P-44.4.1)
Names of cyclic hydrocarbons substituted by chains are formed by substituting chains, saturated or unsaturated, into rings (see P-44.1.2.2). This rule must be strictly applied in the context of preferred IUPAC names. The name ‘toluene’ is retained with no substitution allowed for preferred IUPAC names, but substitution is allowed on both the ring and side chain with certain restrictions (see P-22.1.3) for general nomenclature. The name ‘xylene’ is a preferred IUPAC name, but cannot be substituted and the name ‘mesitylene’ can only be used in general nomenclature and cannot be substituted.
The names ‘styrene’, ‘stilbene’ and ‘fulvene’ are retained only for general nomenclature. Styrene and stilbene can be ring substituted as prescribed in P-31.1.3.4. There is no substitution allowed for ‘fulvene’ (see P-31.1.3.4).
In the 1993 Guide (ref. 2), these parent hydrides were retained but only limited substitution was allowed.
Examples:
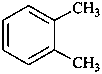
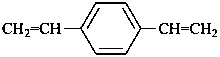
1,4-diethenylbenzene (PIN)
1,4-divinylbenzene
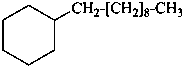
decylcyclohexane (PIN)
(ring preferred to chain, see P-52.2.8)
1-cyclohexyldecane
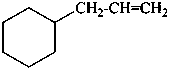
(prop-2-en-1-yl)cyclohexane (PIN)
(ring preferred to chain, see P-52.2.8)
3-cyclohexylprop-1-ene
allylcyclohexane
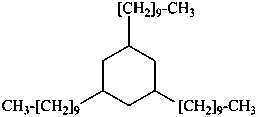
1,3,5-tri(decyl)cyclohexane (PIN)
(not 1,3,5-tris(decyl)cyclohexane)
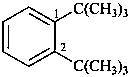
1,2-di-tert-butylbenzene (PIN)
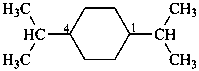
1,4-di(propan-2-yl)cyclohexane (PIN)
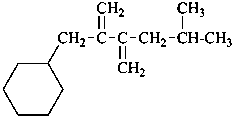
(5-methyl-2,3-dimethylidenehexyl)cyclohexane (PIN)
[not [2-methylidene-3-(2-methylpropyl)but-3-en-1-yl]cyclohexane;
the longer chain is preferred to the shorter unsaturated chains, see P-44.3]
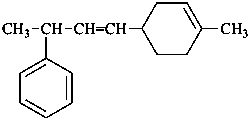
[4-(4-methylcyclohex-3-en-1-yl)but-3-en-2-yl]benzene [PIN; see P-44.3]
{not [1-methyl-3-(4-methylcyclohex-3-en-1-yl)prop-2-en-1-yl]benzene;
the longest chain is preferred to the shorter chains}
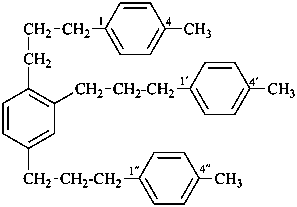
1,1′,1′′-[benzene-1,2,4-triyltri(propane-3,1-diyl)]tris(4-methylbenzene) (PIN)
(multiplicative name, numbering shown, see P-51.3)
1,2,4-tris[3-(4-methylphenyl)propyl]benzene
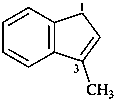
3-methyl-1H-indene (PIN)
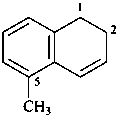
5-methyl-1,2-dihydronaphthalene (PIN)
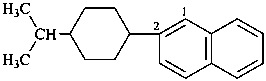
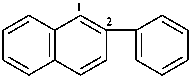
2-[4-(propan-2-yl)cyclohexyl]naphthalene (PIN)
2-(4-isopropylcyclohexyl)naphthalene
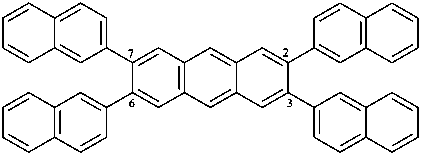
2,3,6,7-tetra(naphthalen-2-yl)anthracene (PIN)
Names of heterocyclic rings or ring systems substituted by chains or rings or ring systems are formed in accordance with the seniority order of rings or ring systems over chains (see P-44.1.2.2) and with the seniority order of rings and ring systems (see P-44.2).
Examples:
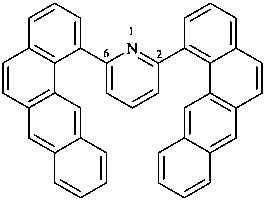
2,6-bis(benzo[a]anthracen-1-yl)pyridine
2,6-di(tetraphen-1-yl)pyridine (PIN)
Halogen compounds in which the halogen atom is in its standard bonding number are always expressed by prefixes in substitutive nomenclature or, as the principal characteristic group or in functional class nomenclature as a separate word.
P-61.3.1 Halogen compounds in which the halogen atom is in its standard bonding number are named in two ways:
(1) by substitutive nomenclature, using the prefixes ‘bromo’, ‘chloro’, ‘fluoro’, and ‘iodo’ and appropriate multiplicative prefixes, as required;(2) by functional class nomenclature, in which names are formed by citing the name of the organic ‘groups’ followed by the class name ‘fluoride’, ‘chloride’, ‘bromide’, or ‘iodide’, as a separate word, preceded, if necessary, by a multiplicative prefix. Functional class names usually are used to denote simple structures, having one kind of halogen, and are not used to name more complex structures. Additive names, such as stilbene dibromide, are not recommended.
Method (1) leads to preferred IUPAC names (see P-51.1).
Examples:
C6H5-CH2-Br
(bromomethyl)benzene (PIN;
no substitution on toluene)
α-bromotoluene
(for toluene substitution rules in general nomenclature; see P-22.1.3)
benzyl bromide
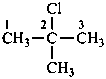
2-chloro-2-methylpropane (PIN)
tert-butyl chloride
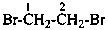
1,2-dibromoethane (PIN)
ethylene dibromide
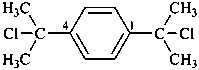
1,4-bis(2-chloropropan-2-yl)benzene (PIN)
1,4-bis(1-chloro-1-methylethyl)benzene
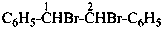
1,1′-(1,2-dibromoethane-1,2-diyl)dibenzene (PIN;
multiplicative name, see P-51.3)
1,2-dibromo-1,2-diphenylethane (substitutive name)
(not stilbene dibromide)
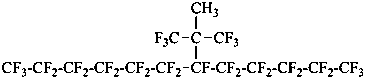
1,1,1,2,2,3,3,4,4,5,5,6,6,7,8,8,9,9,10,10,11,11,12,12,12-pentacosafluoro-7-(1,1,1,3,3,3-hexafluoro-2-methylpropan-2-yl)dodecane (PIN)
1,1,1,2,2,3,3,4,4,5,5,6,6,7,8,8,9,9,10,10,11,11,12,12,12-pentacosafluoro-7-[2,2,2-trifluoro-1-methyl-1-(trifluoromethyl)ethyl]dodecane
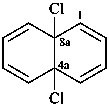
4a,8a-dichloro-4a,8a-dihydronaphthalene (PIN)
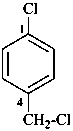
1-chloro-4-(chloromethyl)benzene (PIN)
α,4-dichlorotoluene
(for substitution rules for toluene in general nomenclature; see P-22.1.3)
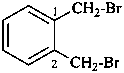
1,2-bis(bromomethyl)benzene (PIN)
α-bromo-2-(bromomethyl)toluene
(for rules on substitution rules of toluene in general nomenclature, see P-22.1.3
[not α,α′-dibromo-o-xylene (no substitution on xylene, see P-22.1.3]
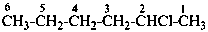
2-chlorohexane (PIN)
hexan-2-yl chloride
1-methylpentyl chloride
F2N-CO-NF2
tetrafluorourea (PIN)
tetrafluorocarbonic diamide
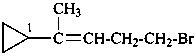
(5-bromopent-2-en-2-yl)cyclopropane (PIN)
(ring preferred to chain, see P-44.1.2.2;
preferred substituent prefix, see P-46.1)
(4-bromo-1-methylbut-1-en-1-yl)cyclopropane
5-bromo-2-cyclopropylpent-2-ene
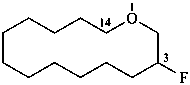
3-fluoro-1-oxacyclotetradecane (PIN)
1-oxacyclotetradecan-3-yl fluoride
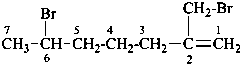
6-bromo-2-(bromomethyl)hept-1-ene (PIN)
2-methylideneheptane-1,6-diyl dibromide
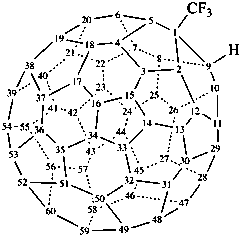
1-(trifluoromethyl)-1,9-dihydro(C60-Ih)[5,6]fullerene (PIN)
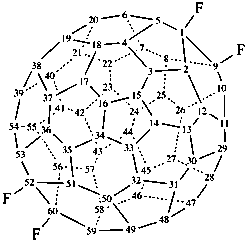
1,9,52,60-tetrafluoro-1,9,52,60-tetrahydro(C60-Ih)[5,6]fullerene (PIN)
(C60-Ih)[5,6]fullerene-1,9,52,60-tetrayl tetrafluoride
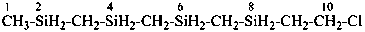
10-chloro-2,4,6,8-tetrasiladecane (PIN)
2,4,6,8-tetrasiladecan-10-yl chloride
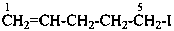
5-iodopent-1-ene (PIN)
pent-4-en-1-yl iodide
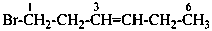
1-bromohex-3-ene (PIN)
hex-3-en-1-yl bromide
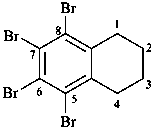
5,6,7,8-tetrabromo-1,2,3,4-tetrahydronaphthalene (PIN)
5,6,7,8-tetrahydronaphthalene-1,2,3,4-tetrayl tetrabromide
(for position of ‘hydro’/‘dehydro’ prefixes in preferred IUPAC names, see P-31.2.1)
P-61.3.2.1 In P-61.3.1, halogen atoms in their standard bonding number are attached to carbon atoms. The halogen atoms can also be attached to heteroatoms. The prefixes ‘bromo’, ‘chloro’, ‘fluoro’, and ‘iodo’ are used to name halogen compounds when the halogen atoms are attached to B, Al, In, Ga, Tl, Si, Ge, Sn, Pb, and Bi.
Examples:
Cl3Si-CH2I
F2Ge=CH2
H2P-PH-Cl
chlorodi(methyl)borane (PIN;
borane is a preselected name; see P-12.2)
dimethylboranyl chloride
trichloro(iodomethyl)silane (PIN;
silane is a preselected name; see P-12.2)
difluoro(methylidene)germane (PIN;
germane is a preselected name; see P-12.2)
chlorodiphosphane (preselected name;
diphosphane is a preselected name see P-12.2)
Examples:
CH3-PH-Cl
methylphosphinous chloride (PIN; see P-67.1.2.5)
chloro(methyl)phosphane
CH3-S-Cl
methyl thiohypochlorite (PIN; see P-67.1.3)
–XO chlorosyl (no longer chloroso), bromosyl, iodosyl, fluorosylExamples:–XO2 chloryl (no longer chloroxy), bromyl, iodyl, fluoryl
–XO3 perchloryl, perbromyl, periodyl, perfluoryl
C6H5-IO
iodosylbenzene (PIN)
P-61.3.4 Retained names
The retained names ‘bromoform’ for HCBr3, ‘chloroform’ for HCCl3, and ‘iodoform’ for HCI3 are acceptable in general nomenclature. Preferred IUPAC names are substitutive names.
Example:
Compounds containing a group =N2 attached to a single carbon atom are named by adding the prefix ‘diazo’ to the name of the parent hydride or functional parent hydride (see also P-74.2.2.2.3).
Examples:
N2CH-CO-O-C2H5
ethyl diazoacetate (PIN)
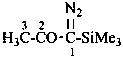
1-diazo-1-(trimethylsilyl)propan-2-one (PIN)
1-diazo-1-(trimethylsilyl)acetone
P-61.5.1 Nitro and nitroso compounds
Compounds containing the –NO2 or –NO group are named by means of the prefixes ‘nitro’ and ‘nitroso’, respectively, unless these groups can be named on the basis of the parent structures nitric and nitrous acids, NO2-OH and NO-OH, respectively, or their corresponding esters, anhydrides, amides and hydrazides. Derivatives of nitric acid and nitrous acids are described in Section P-67. Acid halides and pseudohalides are described in P-67.1.2.5; amides and hydrazides in P-67.1.2.6; salts, esters and anhydrides in P-67.1.3.
Examples:
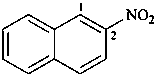
2-nitronaphthalene (PIN)
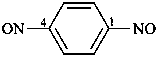
1,4-dinitrosobenzene (PIN)
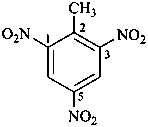
2-methyl-1,3,5-trinitrobenzene (PIN)
2,4,6-trinitrotoluene (for substitution rules for toluene
in general nomenclature, see P-22.1.3
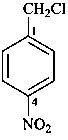
1-(chloromethyl)-4-nitrobenzene (PIN)
α-chloro-4-nitrotoluene (for substitution rules for toluene
in general nomenclature, see P-22.1.3
4-nitrobenzyl chloride
CH3-BH-NO2
methyl(nitro)borane (PIN)
(CH3)3Si-NO2
trimethyl(nitro)silane (PIN)
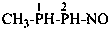
1-methyl-2-nitrosodiphosphane (PIN)
Examples:
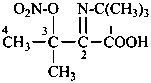
N-methyl-N-nitrosourea (PIN)
Compounds containing the group =N(O)OH are named as derivatives of azinic acid, H2N(O)-OH, a preselected name, and by using the prefix name hydroxy(oxo)-λ5-azanylidene, when a characteristic group having priority for citation as a suffix is present. The use of the prefix ‘aci-nitro’ may be used in general nomenclature (see P-67.1.6).
Example:
Compounds containing the –PO, –PO2, –AsO or –AsO2 are called heterones (see P-64.1.2.2, P-64.4). In the presence of a more senior characteristic group they are described by the compound prefixes oxophosphanyl, dioxo-λ5-phosphanyl, oxoarsanyl, and dioxo-λ5-arsanyl.
Note: In spite of the use of the term ‘phospho’ to describe the –PO2 group as a substituent prefix since 1937, the term ‘phospho’ is widely used in biochemical nomenclature in place of phosphono for designating the –P(O)(OH)2 group linked to a heteroatom, as in phosphocholine and 6-phospho-D-glucose; and as an infix to describe phosphoric diesters, as in glycerophosphocholine. Consequently, in these recommendations the term ‘phospho’, and collaterally the terms phosphoroso, arso, and arsenoso, which are still used in CAS index nomenclature, are no longer used.Examples:
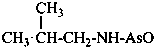
[(2-methylpropyl)amino]arsanone (PIN)
N-(2-methylpropyl)-1-oxoarsanamine
(not N-arsenoso-2-methylpropanamine)
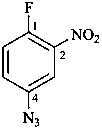
Compounds containing a –N3 (–N=N+=N–) group attached to a parent hydride, are named using substitutive nomenclature and the prefix ‘azido’. This method gives preferred IUPAC names rather than names based on the class name ‘azido’ in functional class nomenclature (see also P-74.2.2.2.2).
Examples:
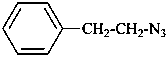
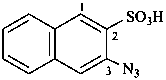
3-azidonaphthalene-2-sulfonic acid (PIN)
This is a change from previous recommendations. Preferred IUPAC names are generated substitutively using the prefix ‘isocyanato’ attached directly to a parent hydride. Previously, functional class names were recommended for this class.
Compounds containing the –N=C=O group attached to a parent hydride structure, are named by using substitutive nomenclature and the prefix ‘isocyanato’. This method leads to preferred IUPAC names rather than names based on functional class nomenclature and the functional class name ‘isocyanate’. Chalcogen analogues are named by inserting the functional replacement infix ‘thio’, ‘seleno’, or ‘telluro’ into the names ‘isocyanate’ or ‘isocyanato’ just after ‘iso’.
Examples:
C6H5-NCS
isothiocyanatobenzene (PIN)
phenyl isothiocyanate
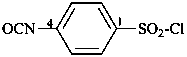
4-isocyanatobenzene-1-sulfonyl chloride (PIN)
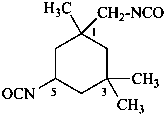
5-isocyanato-1-(isocyanatomethyl)-1,3,3-trimethylcyclohexane (PIN)
3-(isocyanatomethyl)-3,5,5-trimethylcyclohexyl isocyanate
H3Si-NCS
isothiocyanatosilane (PIN;
silane is a preselected name; see P-12.2)
H2B-NCO
isocyanatoborane(PIN;
borane is a preselected name; see P-12.2)
P-61.9 ISOCYANIDES
This is a change in these recommendations. Preferred IUPAC names are formed substitutively using the prefix ‘isocyano’ attached directly to a parent hydride. Previously, functional class names were recommended for this class.
Compounds containing the –NC group attached to a parent hydride structure, are named by substitutive nomenclature and the prefix ‘isocyano’. This method leads to preferred IUPAC names rather than names based on functional class nomenclature and the functional class name ‘isocyanide’.
Examples:
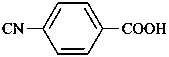
4-isocyanobenzoic acid (PIN)
The structure of fulminic acid was indicated in the 1979 Recommendations (Rule C-833.1, ref. 1) and in the 1993 Recommendations (Rule R-5.7.9.2, ref. 2) as HO-N≡C, and its derivatives were denoted by the class name fulminate and the prefix fulminato. Although consistent with the pseudohalogen cyanate, the structure of this acid in the literature is claimed to be HCNO. Accordingly, the name fulminic acid and that of its substituent group fulminato are not acceptable nor are the names isofulminic acid and isofulminate. The IUPAC preferred name for the structure HCNO is formonitrile oxide (see P-66.5.4.1) and the IUPAC preferred name for its isomer, HO-N=C:, is based on hydroxylamine (see P-68.3.1.1.1).
This is a change from the potentially ambiguous names fulminate and fulminato in previous recommendations to systematic substitutive names.
Examples:
–C≡N=O
(oxo-λ5-azanylidyne)methyl (preferred prefix)
(not isofulminato)
HO-N=C
λ2-methylidenehydroxylamine
N-hydroxy-λ2-methanamine (PIN)
–O-N=C
(λ2-methylideneamino)oxy (preferred prefix)
(not fulminato)
In substitutive names, detachable prefixes (except for ‘hydro’/‘dehydro’ prefixes), are cited in alphanumerical order. Low locants are assigned to:
This is a change from previous recommendations. In these recommendations the prefixes ‘hydro’ and ‘dehydro’ are detachable but not alphabetized with other substituent prefixes. In names, they are cited immediately before the name of the parent hydride, after alphabetized prefixes and before nondetachable prefixes.
(1) the prefixes as a set, and if there is a choice,In functional class nomenclature, names are formed following the order for compound classes (see P-41) and the order of seniority for halides and pseudohalides (see P-41 and P-65.5.2.1) to choose the principal characteristic group. Names formed substitutively rather than functional class names are preferred IUPAC names.(2) to the prefix that is cited first in a name.
P-61.11.1 Low locants are assigned as a set, without regard to kind.
Examples:
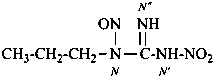
N′-nitro-N-nitroso-N-propylguanidine (PIN)
Examples:
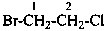
1-bromo-2-chloroethane (PIN)
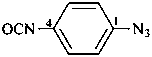
1-azido-4-isocyanatobenzene (PIN)
4-isocyanatophenyl azide
P-62.0 IntroductionP-62.0 INTRODUCTION
P-62.1 General methodology
P-62.2 Amines
P-62.3 Imines
P-62.4 N-Substitution of amines and imines by heteroatoms
P-62.5 Amine oxides, imine oxides and chalcogen analogues
P-62.6 Amine and imine salts
The nomenclature of amines and imines is rich in traditions and a variety of methods have been used for constructing their names (see refs. 1 and 2). The rationalization necessary to define preferred IUPAC names is the appropriate opportunity to establish proper names for amines and imines and retain clear and unambiguous methods for choosing the appropriate parent and naming individual compounds.
Rules C-11.4 and C-811-C-815 in the 1979 Recommendations (ref. 1) are superseded, as well as Rules R-5.4.1–R-5.4.3 in the 1993 Recommendations (ref. 2).
P-62.1 GENERAL METHODOLOGY
The general methodology is based on the following principles:
(a) definitions, as given in the Glossary of Class Names Based on Structure (ref. 23), classify amines and imines unambiguously as follows;P-62.2 AMINES
(1) monoamines are compounds formally derived from ammonia (NH3) by replacing one, two, or three of its hydrogen atoms by one, two, or three hydrocarbyl groups by single bonds, thus having the general structures R-NH2 (primary amines), R2NH (secondary amines), R3N (tertiary amines);(b) amines are senior to imines in the seniority order of classes;(2) imines are compounds having the structure R2C=NR (R = H or hydrocarbyl), corresponding either to ketimines, RR′C=NR′′ or to aldimines, RCH=NR′;
(c) methods for naming amines and imines will be restricted to a minimum, preference being given to the substitutive method using the suffixes ‘amine’ and ‘imine’;
(d) a minimum of traditional names will be retained;
(e) polyamines are further classified as follows;
(1) simple polyamines are compounds in which all amino groups are attached to the same parent hydride;(2) complex polyamines are compounds in which a choice between two or more parent hydrides must be made.
P-62.2.1 Primary amines
P-62.2.1.1 Retained names
P-62.2.1.1.1 Aniline, for C6H5-NH2 , is the only name for a primary amine retained as a preferred IUPAC name for which full substitution is permitted on the ring and the nitrogen atom. It is a Type 2a retained name; for the rules of substitution see P-15.1.8.2. Substitution is limited to substituent groups cited as prefixes in accordance with the seniority of functional groups explicitly expressed or implied in the functional parent compound name. The name benzenamine may be used in general nomenclature. The prefix name ‘anilino’ is retained as the preferred prefix for C6H5-NH– with full substitution allowed. The name ‘phenylamino’ may be used in general nomenclature.
Examples:
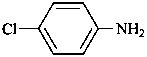
4-chloroaniline (PIN)
4-chlorobenzenamine
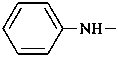
anilino (preferred prefix)
phenylamino
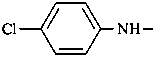
4-chloroanilino (preferred prefix)
(4-chlorophenyl)amino
Examples:
4-methylanilino (preferred prefix)
(4-methylphenyl)amino
(not p-toluidino)
(1) by adding the suffix ‘amine’ to the name of the parent hydride;Note: Amine is not a true preselected parent hydride. In these recommendations, it is considered as a ‘pseudo’ parent hydride, based on the premise that this method originated by modification of a functional class name based on the class name amine, for example, ethyl amine. The space in the functional class name is eliminated to form the current name ethylamine.(2) by adding the name of the substituent group R− to the parent hydride ‘azane’;
(3) by adding the name of the substituent group R− to the term ‘amine’ used as a preselected parent hydride name for NH3; this method is used only with monoamines.
Method (1) leads to preferred IUPAC names.
Examples:
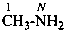
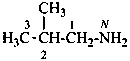
(1) 2-methylpropan-1-amine (PIN)
(2) (2-methylpropyl)azane
(3) (2-methylpropyl)amine
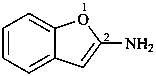
1-benzofuran-2-amine (PIN)
(1-benzofuran-2-yl)azane
(1-benzofuran-2-yl)amine
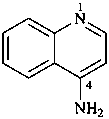
quinolin-4-amine (PIN)
(quinolin-4-yl)azane
(quinolin-4-yl)amine
4-quinolylamine
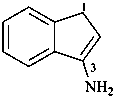
1H-inden-3-amine (PIN)
(1H-inden-3-yl)azane
(1H-inden-3-yl)amine
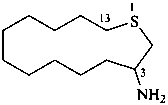
1-thiacyclotridecan-3-amine (PIN)
(1-thiacyclotridecan-3-yl)azane
(1-thiacyclotridecan-3-yl)amine
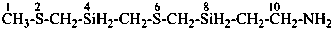
2,6-dithia-4,8-disiladecan-10-amine (PIN)
(2,6-dithia-4,8-disiladecan-10-yl)azane
(2,6-dithia-4,8-disiladecan-10-yl)amine
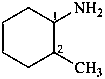
2-methylcyclohexan-1-amine (PIN)
(2-methylcyclohexyl)azane
(2-methylcyclohexyl)amine
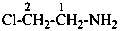
2-chloroethan-1-amine (PIN)
(2-chloroethyl)azane
(2-chloroethyl)amine
When attached to heteroatoms, amino groups are expressed as suffixes when representing the principal characteristic group.
Examples:
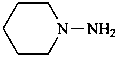
(CH3)3Si-NH2
1,1,1-trimethylsilanamine (PIN)
(trimethylsilyl)azane
(trimethylsilyl)amine
P-62.2.2.1 Symmetrical and unsymmetrical secondary and tertiary amines are named only by the same methods described in P-62.2.1.2.
(1) substitutively using the retained name ‘aniline’ or the suffix ‘amine’ and the name of a parent hydride with further N-substitution;Method (1) generates preferred IUPAC names. Functional parent names like diethylamine and triethylamine are deprecated. The prefixes in names of such secondary and tertiary amines formed by method (3) are set off by parentheses to distinguish them from these deprecated names.(2) substitutively, by prefixing, in alphabetical order when required, the name(s) of the substituent group(s) R, R′ or R′′ to the parent hydride name ‘azane’. In order to avoid ambiguity, the second prefix in a secondary amine, and the second and the third prefixes in a tertiary amines must be enclosed in parentheses when these prefixes denote simple substituents.
(3) substitutively, by prefixing, in alphabetical order when required, the name(s) of the substituent group(s) R, R′ or R′′ to the parent hydride name ‘amine’. In order to avoid ambiguity, the second prefix in a secondary amine, and the second and the third prefixes in a tertiary amines must be enclosed in parentheses when these prefixes denote simple substituents.
Examples:
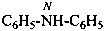
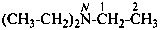
(1) N,N-diethylethanamine (PIN)
(2) triethylazane
(3) (triethyl)amine
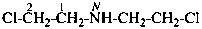
2-chloro-N-(2-chloroethyl)ethan-1-amine (PIN)
bis(2-chloroethyl)azane
bis(2-chloroethyl)amine
(not 2,2′-dichlorodiethylamine)
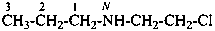
(1) N-(2-chloroethyl)propan-1-amine (PIN)
(2) (2-chloroethyl)(propyl)azane
(3) (2-chloroethyl)(propyl)amine
[not N-(2-chloroethyl)propylamine]
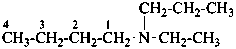
(1) N-ethyl-N-propylbutan-l-amine (PIN)
(2) butyl(ethyl)(propyl)azane
(3) butyl(ethyl)(propyl)amine
(not N-ethyl-N-propylbutylamine)
H3Si-NH-SiH3
(1) N-silylsilanamine (preselected name)
(2) disilylazane
(3) (disilyl)amine
(not disilazane; see P-21.2.3.1)
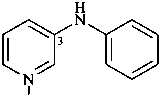
(1) N-phenylpyridin-3-amine (PIN)
(2) phenyl(pyridin-3-yl)azane
(3) phenyl(pyridin-3-yl)amine
[not N-(pyridin-3-yl)aniline]
Names of amines formed substitutively by using the retained name aniline or the suffix ‘amine’ are based on a principal chain and a senior ring system (see P-44.1). When a choice for parent hydride is possible between a ring and a chain, the ring is preferred. In names using ‘amine’ as a parent hydride, substituent groups expressed as prefixes are cited in alphanumerical order; the prefix(es) immediately preceding the term ‘amine’ is (are) enclosed in parentheses.
Examples:
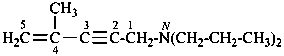
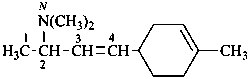
N,N-dimethyl-4-(4-methylcyclohex-3-en-1-yl)but-3-en-2-amine (PIN)
dimethyl[4-(4-methylcyclohex-3-en-1-yl)but-3-en-2-yl]azane
dimethyl[4-(4-methylcyclohex-3-en-1-yl)but-3-en-2-yl]amine
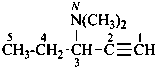
N,N-dimethylpent-1-yn-3-amine (PIN)
dimethyl(pent-1-yn-3-yl)azane
dimethyl(pent-1-yn-3-yl)amine
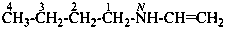
N-ethenylbutan-1-amine (PIN)
butyl(ethenyl)azane
butyl(ethenyl)amine
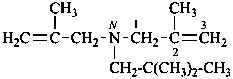
N-(2,2-dimethylpropyl)-2-methyl-N-(2-methylprop-2-en-1-yl)prop-2-en-1-amine (PIN)
(2,2-dimethylpropyl)bis(2-methylprop-2-en-1-yl)azane
(2,2-dimethylpropyl)bis(2-methylprop-2-en-1-yl)amine
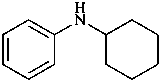
N-cyclohexylaniline (PIN)
cyclohexyl(phenyl)azane
cyclohexyl(phenyl)amine
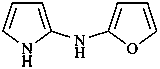
N-(furan-2-yl)-1H-pyrrol-2-amine (PIN)
(furan-2-yl)(1H-pyrrol-2-yl)azane
2-furyl(1H-pyrrol-2-yl)azane
(furan-2-yl)(1H-pyrrol-2-yl)amine
2-furyl(1H-pyrrol-2-yl)amine
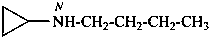
N-butylcyclopropanamine (PIN)
butyl(cyclopropyl)azane
(not N-cyclopropylbutan-1-amine)
butyl(cyclopropyl)amine
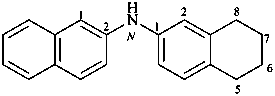
N-(5,6,7,8-tetrahydronaphthalen-2-yl)naphthalen-2-amine (PIN)
2-naphthyl(5,6,7,8-tetrahydro-2-naphthyl)azane
2-naphthyl(5,6,7,8-tetrahydro-2-naphthyl)amine
[not 5,6,7,8-tetrahydrodi(2-naphthyl)amine]
Examples:
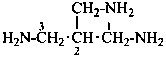
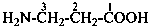
3-aminopropanoic acid (PIN)
3-azanylpropanoic acid
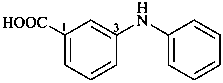
3-anilinobenzoic acid (PIN)
3-(phenylamino)benzoic acid
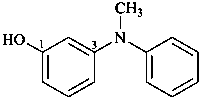
3-(N-methylanilino)phenol (PIN)
3-[methyl(phenyl)amino]phenol
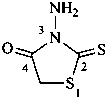
3-amino-2-sulfanylidene-1,3-thiazolidin-4-one (PIN)
3-amino-2-thioxo-1,3-thiazolidin-4-one
(see also P-64.6.1)
Examples:
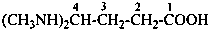
(H3Si)2N-CH2-COOH
N,N-disilylglycine
(disilylamino)acetic acid
(disilylazanyl)acetic acid
[not (disilazan-2-yl)acetic acid]
H3Si-HN-SiH2–
(silylamino)silyl (preselected prefix)
(silylazanyl)silyl
(not disilazan-1-yl)
P-62.2.4.1 Simple polyamines are compounds in which all amino groups are attached to the same parent hydride
P-62.2.4.1.1 There are no retained names for simple polyamines that are used as preferred IUPAC names. However, in general nomenclature the name ‘benzidine’ may be used but only for the 4,4′-isomer, with substitution allowed as described in P-15.1.8.2 for a Type 2 retained name. The prefix ‘benzidino’ is retained with full substitution.
Examples:
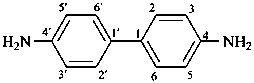
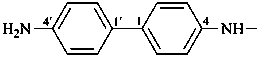
benzidino
(4′-amino[1,1′-biphenyl]-4-yl)amino (preferred prefix)
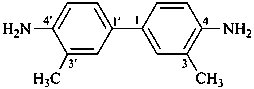
3,3′-dimethylbenzidine
3,3′-dimethyl[1,1′-biphenyl]-4,4′-diamine (PIN)
P-62.2.4.1.2 Two or more ‘amine’ groups attached to the same parent hydride are indicated by an appropriate multiplying numerical prefix ‘di’, ‘tri’, ‘tetra’, etc. The terminal letter ‘a’ of a numerical prefix is elided before the suffix amine, i. e., ‘tetramine’, not ‘tetraamine’. Numerical locants, including ‘1’ in the case of amines derived from mononuclear parent hydrides, are used to denote substitution on atoms of the parent hydride and ‘N’ locants for substitution on the nitrogen atom for amines named by method (1). Method (2) is used only for monoamines.
Examples:
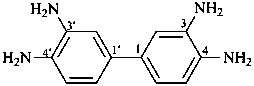
[1,1′-biphenyl]-3,3′,4,4′-tetramine (PIN;
note the elision of ‘a’ from ‘tetra’ in ‘tetramine’)
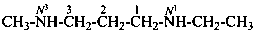
N1-ethyl-N3-methylpropane-1,3-diamine (PIN)
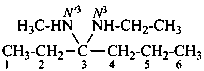
N3-ethyl-N′3-methylhexane-3,3-diamine (PIN)
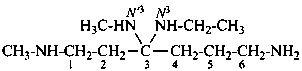
N3-ethyl-N1,N′3-dimethylhexane-1,3,3,6-tetramine (PIN)
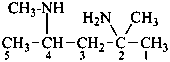
N4,2-dimethylpentane-2,4-diamine (PIN)
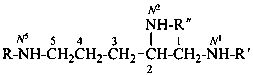
R = R′ = R′′ = H pentane-1,2,5-triamine (PIN)
R = R′ = H; R′′ = –CH3 N2-methylpentane-1,2,5-triamine (PIN)
R = H; R′ = –CH3; R′′ = –CH2-CH3 N2-ethyl-N1-methylpentane-1,2,5-triamine(PIN)
In complex polyamines a senior parent amine structure must be chosen. The senior parent amine structure is chosen in accordance with the choice of a principal chain or a senior ring or ring system, as described in P-44, or the preferred IUPAC name must be chosen in accordance with P-45. Alphanumerical order is applied when necessary. Multiplicative nomenclature, skeletal replacement (‘a’) nomenclature, or phane nomenclature are used when the conditions required by these types of nomenclature are fulfilled.
Examples:
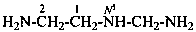
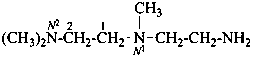
N1-(2-aminoethyl)-N1,N2,N2-trimethylethane-1,2-diamine (PIN; numbering shown)
(the most substituted diamine is chosen as parent structure; P-45.2.1)
[not N1,N1-dimethyl-2,2′-(methylazanediyl)di(ethan-1-amine);
even in general nomenclature the parent name must be a diamine;
ethanamine is a monoamine]
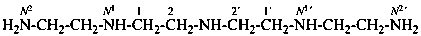
N1,N1′-[azanediyldi(ethane-2,1-diyl)]di(ethane-1,2-diamine) [PIN;
the parent structure is a diamine and multiplicative nomenclature (see P-15.3; P-51.3; P-62.2.5)
allows four amine characteristic groups to be included in the name; numbering shown]
N1-(2-aminoethyl)-N2-{2-[(2-aminoethyl)amino]ethyl}ethane-1,2-diamine (substitutive name)
[not 2,2′-azanediylbis[N-(2-aminoethyl)ethan-1-amine] [substitutive name;
even in general nomenclature, the parent structure must be a diamine; ethanamine is a monoamine]
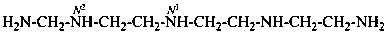
N1-{2-[(2-aminoethyl)amino]ethyl}-N2-(aminomethyl)ethane-1,2-diamine (PIN)
[not N2-(aminomethyl)-N1-{2-[(2-aminoethyl)amino]ethyl}ethane-1,2-diamine;
‘aminoethyl’ precedes ‘aminomethyl’ in alphanumerical order; P-14.5]
[not N1-(2-aminoethyl)-N2-(aminomethyl)-2,2′-azanediyldi(ethan-1-amine);
the preferred IUPAC name must be a diamine]
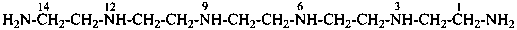
3,6,9,12-tetraazatetradecane-1,14-diamine (PIN)
(skeletal replacement (‘a’) name; see P-15.4)
N1,N2-bis{2-[(2-aminoethyl)amino]ethyl}ethane-1,2-diamine (substitutive name)
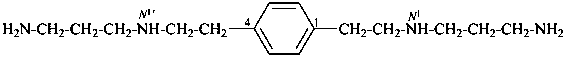
N1,N1′-[1,4-phenylenedi(ethane-2,1-diyl)]di(propane-1,3-diamine) (PIN;
[multiplicative nomenclature (see P-15.3; P-51.3; P-62.2.5)
allows four amine characteristic groups to be included in the name)
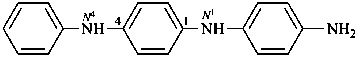
N1-(4-aminophenyl)-N4-phenylbenzene-1,4-diamine (PIN)
(maximum number of substituents cited as prefixes; see P-45.2.1)
[not N1-(4-anilinophenyl)benzene-1,4-diamine]
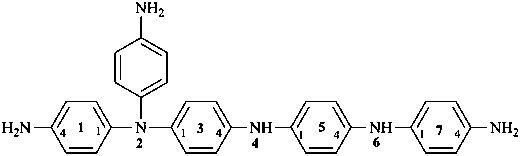
2-(4-aminophenyl)-2,4,6-triaza-1,7(1),3,5(1,4)-tetrabenzenaheptaphane-14,74-diamine (PIN)
(four benzene rings and a total of seven phane nodes justify a phane name; see P-52.2.5)
N1,N1-bis(4-aminophenyl)-N4-{4-[(4-aminophenyl)amino]phenyl}benzene-1,4-diamine
(preferred substitutive name; maximum number of substituent prefixes)
N1-(4-aminophenyl)-N1,N1′-[azanediyldi(4,1-phenylene)]di(benzene-1,4-diamine)
(multiplicative name; applicable only in general nomenclature)
N4-[4-(4-aminoanilino)phenyl]-N1,N1-bis(4-aminophenyl)benzene-1,4-diamine
P-62.2.5.1 The prefixes ‘nitrilo’ for –N< and ‘azanediyl’ for –NH– (also written HN<) are recommended for use in multiplicative nomenclature (see P-15.3). The prefix ‘imino’ is reserved to denote only the divalent substituent group =NH. Multiplicative names are preferred to those formed by substitutive nomenclature when all conditions are fulfilled for the application of multiplicative nomenclature (see P-51.3).
Examples:

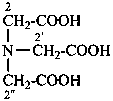
2,2′,2′′-nitrilotriacetic acid
N,N-bis(carboxymethyl)glycine
The use of priming on the italic letter N to differentiate among different nitrogen atoms of parent structures requires special methods to indicate the attachment of identical parent structures to the multiplying substituent group through a nitrogen atom.
(1) Multiplied parent structures containing one nitrogen atom. The nitrogen atoms attached to the central substituent group are denoted by the symbols N, N′, N′′, etc.P-62.2.5.3 For parent structures with multiple nitrogen atoms, such as di- or tricarboximidamides (see P-66.4), and cyclophanamines, even more complicated locant structures are required, such as primed letter locants with superscript numbers or with superscripted superscript numbers.Example:
(2) Multiplied parent structures containing two nitrogen atoms. 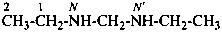
N,N′-methylenediethanamine (PIN)
N,N′-diethylmethanediamine
(i) The symbols N,N′ are used for the ‘unprimed’ parent structure and N′′,N′′′ for the ‘primed parent structure‘.Example:
(ii) A combination of unprimed and primed numerical and letter locants is used as needed (see P-16.9.3). The numerical locant of the position on the parent structure at which the nitrogen atom is attached is cited as a superscript to the letter locant N, for example, N1, N1′, etc. A second identical parent structure has primed locants, i.e., 1′, 2′..., hence the nitrogen locant is N1′. 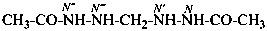
N′,N′′′-methylenediacetohydrazide (PIN)
Examples:
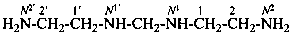
N1,N1′-methylenedi(ethane-1,2-diamine) (PIN)
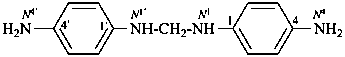
N1,N1′-methylenedi(benzene-1,4-diamine) (PIN)
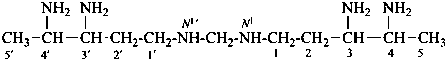
N1,N1′-methylenedi(pentane-1,3,4-triamine) (PIN)
Example:
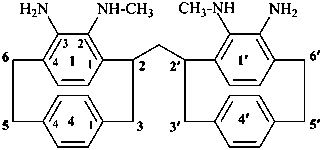
P-62.2.6.1 General methodology
When there is a choice for numbering, the starting point and the direction of numbering of a compound are chosen so as to give lowest locants to the following structural features (if present) considered successively in the order given until a decision is reached (see also P-14.4).
(a) fixed numbering (naphthalene, bicyclo[2.2.2]octane, etc.);P-62.2.6.2 Modification of the degree of saturation/unsaturation of primary amines(b) heteroatoms in heterocycles and in acyclic parent structures;
(c) indicated hydrogen [for unsubstituted compounds; a higher locant may be needed at another position to provide for a substituent suffix in accordance with the structural feature (d)];
(d) principal group named as suffix;
(e) added indicated hydrogen (consistent with the structure of the compound and in accordance with further substitution);
(f) saturation/unsaturation (‘hydro’/‘dehydro’ prefixes) or unsaturation (‘ene’, ‘yne’ endings);
(g) substituents named as prefixes (low locants are allocated for substituents regardless of kind; then, if necessary, in the order of citation).
Criteria (d), (e) and (f) described in the general methodology (P-62.2.6.1) are used.
Examples:
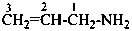
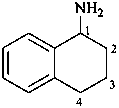
1,2,3,4-tetrahydronaphthalen-1-amine (PIN)
(1,2,3,4-tetrahydronaphthalen-1-yl)azane
(1,2,3,4-tetrahydronaphthalen-1-yl)amine
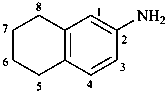
5,6,7,8-tetrahydronaphthalen-2-amine (PIN)
(5,6,7,8-tetrahydronaphthalen-2-yl)azane
(5,6,7,8-tetrahydronaphthalen-2-yl)amine
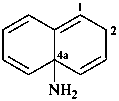
naphthalen-4a(2H)-amine (PIN)
(naphthalen-4a(2H)-yl)azane (naphthalen-4a(2H)-yl)amine
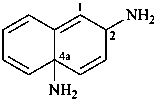
naphthalene-2,4a(2H)-diamine (PIN)
2,4a-dihydronaphthalene-2,4a-bis(azane) (see P-58.2)
2,4a-dihydronaphthalene-2,4a-diamine (see P-58.2)
naphthalene-2,4a(2H)-bis(azane)
Imines are characterized by a double bond between a carbon atom and a nitrogen atom. Thus, N-substituted imines, R-CH=N-R′ or R(R′)C=N-R′′, must be classified as imines and not as amines in spite of the fact that there is a single bond between a carbon atom and the nitrogen atom; amines must have three single bonds linked to at least one carbon atom (see P-62.1). Imines must have a double bond between a carbon atom and the nitrogen. Compounds having the general structure R-CH=NR′ or R(R′)C=NR′′ are called generically ‘aldimines’ and ‘ketimines’, respectively.
P-62.3.1 Substitutive names for imines
P-62.3.1.1 All imines are named substitutively using the suffix ‘imine’; the presence of several ‘imine’ characteristic groups is denoted by the numerical multiplying prefixes ‘di’, ‘tri’, etc. When there is a choice for numbering, the methodology described in P-62.2.4 for amines is recommended to generate preferred IUPAC names
Examples:
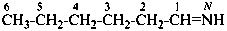
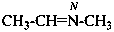
N-methylethanimine (PIN)
[not N-ethylidenemethanamine;
nor N-ethylidene(methyl)amine]
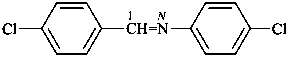
N,1-bis(4-chlorophenyl)methanimine (PIN)
(cf. the following example)
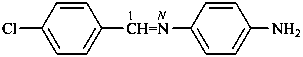
4-{[(4-chlorophenyl)methylidene]amino}aniline (PIN)
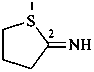
thiolan-2-imine (PIN)
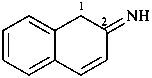
naphthalen-2(1H)-imine (PIN)
1,2-dihydronaphthalen-2-imine (see P-58.2.5)
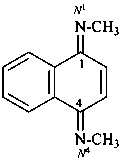
N1,N4-dimethylnaphthalene-1,4-diimine (PIN; see also P-16.9.2)
N,N′-dimethylnaphthalene-1,4-diimine (see also P-58.2.2.3)
N,N′-dimethyl-1,4-dihydronaphthalene-1,4-diimine
[not N,N′-dimethyl-1,4-naphthoquinone diimine;
two suffixes of different kinds are incompatible]
[not N,N′-(naphthalene-1,4-diylidene)bis(methanamine)]
[not N,N′-(naphthalene-1,4-diylidene)bis(methylamine)]
[not dimethyl(naphthalene-1,4-diylidene)bis(amine)]
Examples:
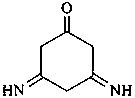
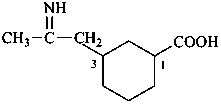
3-(2-iminopropyl)cyclohexane-1-carboxylic acid (PIN)
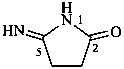
5-iminopyrrolidin-2-one (PIN)
(not 5-imino-2-pyrrolidone)
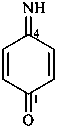
4-iminocyclohexa-2,5-dien-1-one (PIN)
(not p-benzoquinone monoimine)
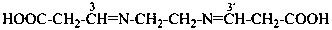
3,3′-[ethane-1,2-diylbis(azanylylidene)]dipropanoic acid (PIN)
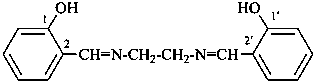
2,2′-[ethane-1,2-diylbis(azanylylidenemethanylylidene)]diphenol (PIN)
Compounds containing the group X=NH, where X is a heteroatom and =NH the principal characteristic group, are named as imines; the prefix ‘imino’ is used to express the =NH group when another characteristic group has seniority over imines.
Examples:
(CH3)2Si=N-C6H5
1,1-dimethyl-N-phenylsilanimine (PIN)
(silane is a preselected name, see P-12.2)
CH3-N=SiH-CH2-CO-O-CH3
methyl [(methylimino)silyl]acetate (PIN)
(silyl is a preselected prefix, see P-12.2)
The hypothetical compound HN=C=NH is named systematically ‘methanediimine’. Its derivatives are named as substitution products thereof. These names are preferred to those based on the retained name ‘carbodiimide’, which now should be used only as a class name.
Example:
Traditionally, substitution on the nitrogen atom of amines and imines was allowed for all characteristic groups cited as prefixes (see Table 5.1). This approach is maintained in these recommendations, unless a higher class is formed that must be named in accordance with the seniority of classes (see P-41).
This new rule is applied to prefixes such as Cl and other halogen atoms, –BrO and other acyl similar groups, –NO, –NO2, –OR, –SO2-R, –SO-R, and even –OH groups and chalcogen analogues.
This is a change. In accordance with the seniority of classes (see P-41), compounds such as R-NH-Cl, R-NH-NO, and R-NH-NO2 are now named as derivatives of amides (see P-67.1.2.6). Compounds such as R-NH-OH are named as N-derivatives of the senior amine (see P-68.3.1.1.1).
Substitution of amines is permitted by –OR , –SR, –SeR, and –TeR groups, where R is an alkyl or aryl substituent group.
Examples:
CH3-CH2-NH-OH
N-hydroxyethanamine (PIN)
N-ethylhydroxylamine
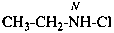
ethylhypochlorous amide (PIN)
N-chloroethanamine
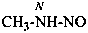
methylnitrous amide (PIN)
N-nitrosomethanamine
CH3-N(NO2)2
methyl(nitro)nitramide (PIN)
N,N-dinitromethanamine
CH3-NH-BrO
methylbromous amide (PIN)
N-bromosylmethanamine
Amine oxides, imine oxides, and their chalcogen analogues are named:
(1) by functional class nomenclature using the class names ‘oxide’, ‘sulfide’, ‘selenide’, and ‘telluride’ provided that unambiguous superscripted N locants can be used, if necessary ;Method (1) is used when one amine or imine oxide is present. Because of the zwitterionic nature of a nitrogen oxide, amine and imine oxides are placed with zwitterions in the order of compound classes (see P-41). Thus, amine and imine oxides are named by method (1) and all other amino groups, if present, are named as substituent groups by using the prefix ‘amino’. Method (2) is used when the oxide is on a nitrogen atom of a substituent group; the locant N is used before the term ‘oxide’ when locants are present in the name of the amine.(2) by use of prefixes derived from the parent name λ5-azane;
(3) as zwitterions (see P-74.2.1.2).
Method (1) or (2), as appropriate, leads to preferred IUPAC names as illustrated below. Names of zwitterions are described in P-74.2.1.2.
Examples:
CH2=N(O)Cl
(1) N-chloromethanimine N-oxide (PIN)
(3) [chloro(methylidene)azaniumyl]oxidanide (see also P-74.2.1.2)
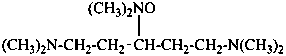
1,5-bis(dimethylamino)-N,N-dimethylpentan-3-amine N-oxide (PIN)
(not N1,N1,N3,N3,N5,N5-hexamethylpentane-1,3,5-triamine N3-oxide;
the N-oxide is classified as a zwitterion and it is the preferred characteristic group)
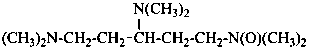
3,5-bis(dimethylamino)-N,N-dimethylpentan-1-amine N-oxide (PIN)
(not N1,N1,N3,N3,N5,N5-hexamethylpentane-1,3,5-triamine N1-oxide;
the N-oxide is classified as a zwitterion and it is the preferred characteristic group)
(CH3)2N-CH2-CH2-CH2-CH2-CH2-N(O)(CH3)2
5-(dimethylamino)-N,N-dimethylpentane-1-amine N-oxide (PIN)
(not N1,N1,N5,N5-tetramethylpentane-1,5-diamine N1-oxide;
the N-oxide is classified as a zwitterion and it is the preferred characteristic group)
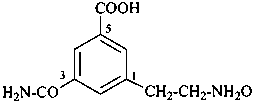
(1) 2-(3-carbamoyl-5-carboxyphenyl)ethan-1-amine N-oxide (PIN)
3-(2-aminoethyl)-5-carbamoylbenzoic acid N3-oxide
(3) {[2-(3-carbamoyl-5-carboxyphenyl)ethyl]azaniumyl}oxidanide (see also P-74.2.1.2)
•CH2-CH2-NH2O
(2) 2-(oxo-λ5-azanyl)ethyl (PIN)
(3) 2-(oxidoazaniumyl)ethyl
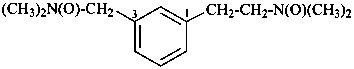
(2) 2-(3-{[dimethyl(oxo)-λ5-azanyl]methyl}phenyl)-N,N-dimethylethan-1-amine N-oxide (PIN)
2-{3-[(dimethylamino)methyl]phenyl}-N,N-dimethylethan-1-amine N1,N3-dioxide
(3) 2-(3-{[dimethyl(oxido)azaniumyl]methyl}phenyl)-N,N-dimethylethan-1-amine N-oxide
(see also P-74.2.1.2)
(CH3-CH2)3NS
(1) N,N-diethylethanamine N-sulfide (PIN)
(triethyl)amine sulfide
(3) (triethylazaniumyl)sulfanide (see also P-74.2.1.2)
P-62.6.1 Cation and anion names
Salts of tetravalent nitrogen R4N+ X– (where one R group represents the parent hydride of the amine or imine and the other groups are hydrogen atoms or substituent groups) are named by one of the following methods:
(1) by adding the suffix ‘ium’ to the name of the amine or imine, with elision of the terminal letter ‘e’, if present, substituent groups being cited as prefixes, and the name of the anion added as a separate word;Method (1) leads to preferred IUPAC names.(2) by substituting the parent hydride ‘azanium’, NH4+;
(3) by substituting the parent hydride ‘ammonium’, NH4+, for quaternary salts only.
Examples:
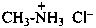
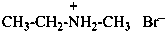
(1) N-methylethanaminium bromide (PIN)
(2) ethyl(methyl)azanium bromide
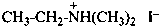
(1) N,N-dimethylethanaminium iodide (PIN)
(2) ethyldi(methyl)azanium iodide
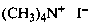
(1) N,N,N-trimethylmethanaminium iodide (PIN)
(2) tetramethylazanium iodide
(3) tetramethylammonium iodide
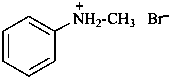
(1) N-methylanilinium bromide (PIN)
N-methylbenzenaminium bromide
(2) methyl(phenyl)azanium bromide
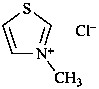
(1) 3-methyl-1,3-thiazol-3-ium chloride (PIN)
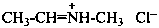
(1) N-methylethaniminium chloride (PIN)
(2) ethylidene(methyl)azanium chloride
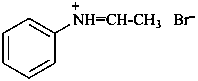
(1) N-phenylethaniminium bromide (PIN)
(2) ethylidene(phenyl)azanium bromide
When the above rule cannot be applied because the structure is indefinite, amine and imine salts are named as organic-inorganic adducts, see P-14.8.2. Formulas for these adducts are written as described in P-14.8. Names are constructed in the order that the formulas are written. Preferred IUPAC names cannot be assigned to salts that include inorganic acids pending the development of rules for choosing preferred IUPAC names for inorganic substances.
Examples:
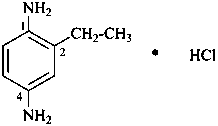
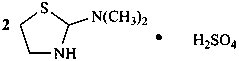
N,N-dimethyl-1,3-thiazolidin-2-amine—sulfuric acid (2/1)
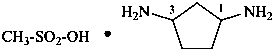
methanesulfonic acid—cyclopentane-1,3-diamine (1/1) (PIN)
P-63.0 IntroductionP-63.0 INTRODUCTION
P-63.1 Hydroxy compounds and chalcogen analogues
P-63.2 Ethers and chalcogen analogues
P-63.3 Peroxides and chalcogen analogues
P-63.4 Hydroperoxides (peroxols) and chalcogen analogues
P-63.5 Cyclic ethers, sulfides, selenides, and tellurides
P-63.6 Sulfoxides and sulfones
P-63.7 Polyfunctional compounds
P-63.8 Salts of hydroxy compounds, hydroperoxy compounds and their chalcogen analogues
Traditionally, hydroxy compounds are compounds having one or more hydroxy groups attached to carbon atoms. Alcohols, phenols, ‘enols’ and ‘ynols’ are recognized as important classes of hydroxy compounds. The category is extended so as to include compounds having one or more hydroxy groups attached to atoms other than carbon without being classified as acids as defined in the seniority of classes. For instance, H3Si-OH is classified and named as a hydroxy compound, silanol, but Si(OH)4 is classified and named as an acid, silicic acid.
The suffix ‘peroxol’ is now introduced to name the group –OOH, formerly named by functional class nomenclature as ‘hydroperoxide’. Chalcogen analogues are names by suffixes such as ‘thioperoxol’, ‘dithioperoxol’, ‘selenoperoxol’, and ‘selenothioperoxol’.
Rules on hydroxy compounds (alcohols and phenols), ethers, hydroperoxides, peroxides, and their chalcogen analogues, discussed as Rules C-201 to C-218 in the 1979 Recommendations (ref. 1) and Rule R-5.5 in the 1993 Recommendations (ref. 2) are superseded by the corresponding rules described in this section, P-63.
P-63.1 HYDROXY COMPOUNDS AND CHALCOGEN ANALOGUES
Names generated substitutively are preferred IUPAC names rather than functional class names or retained names, with the exception of the retained name ‘phenol’ that can be fully substituted. Functional class names are traditional names that are restricted today to alcohols, R-OH, where the R– group is a simple aliphatic or alicyclic group.
P-63.1.1 Retained namesP-63.1.1 Retained names
P-63.1.2 Systematic names of alcohols, phenols, enols, and ynols
P-63.1.3 Heterols
P-63.1.4 Substitutive nomenclature, prefix mode
P-63.1.5 Sulfur, selenium and tellurium analogues of hydroxy compounds
P-63.1.1.1 Only one name is retained, phenol, for C6H5-OH, both as a preferred name and for general nomenclature. The structure is substitutable at any position. Locants 2, 3, and 4 are recommended, not o, m, and p.
Examples:
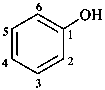
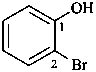
2-bromophenol (PIN)
(not o-bromophenol)
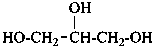
glycerol
propane-1,2,3-triol (PIN)
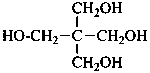
pentaerythritol
2,2-bis(hydroxymethyl)propane-1,3-diol (PIN)
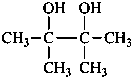
pinacol
2,3-dimethylbutane-2,3-diol (PIN)
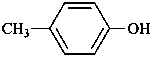
cresol (p-isomer shown ; also o- and m-isomers)
4-methylphenol (PIN)
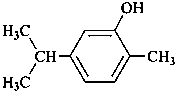
carvacrol
2-methyl-5-(propan-2-yl)phenol (PIN)
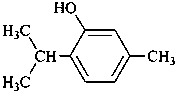
thymol
5-methyl-2-(propan-2-yl)phenol (PIN)
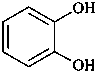
pyrocatehol
benzene-1,2-diol (PIN)
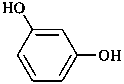
resorcinol
benzene-1,3-diol (PIN)
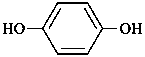
hydroquinone
benzene-1,4-diol (PIN)
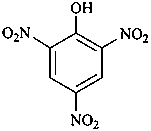
picric acid
2,4,6-trinitrophenol (PIN)
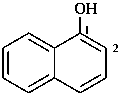
1-naphthol
naphthalen-1-ol (PIN)
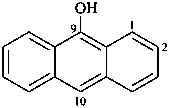
9-anthrol
anthracen-9-ol (PIN)
Hydroxy compounds are named in three ways:
(1) substitutively, using the suffix ‘ol’ and the prefix ‘hydroxy’. The presence of several ‘ol’ characteristic groups is denoted by the numerical multiplying prefixes ‘di’, ‘tri’, etc.; the final letter ‘a’ in a multiplying prefix is elided before the suffix ‘ol’. Rule P-44 is applied when a principal chain or a senior ring system must be chosen. When there is a choice for numbering, the starting point and the direction of numbering of a compound are chosen so as to give lowest locants to the ‘ol’ suffixes;Examples:(2) by functional class nomenclature and the class term ‘alcohol’;
(3) as assemblies of identical units by multiplicative nomenclature when the conditions for its use are fulfilled (see P-51.3)
Method (1) generates preferred IUPAC names. Names as assemblies of identical units, method (3), are preferred to those that are formed by simple substitution (P-51.1.5).
CH3-OH
(1) methanol (PIN)
(2) methyl alcohol
(CH3)3C-OH
(1) 2-methylpropan-2-ol (PIN)
(2) tert-butyl alcohol
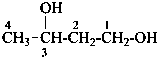
(1) butane-1,3-diol (PIN)
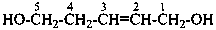
(1) pent-2-ene-1,5-diol (PIN)
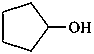
(1) cyclopentanol (PIN)
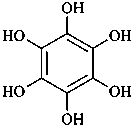
(1) benzenehexol (PIN)
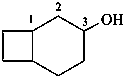
(1) bicyclo[4.2.0]octan-3-ol (PIN)
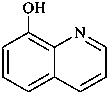
(1) quinolin-8-ol (PIN)
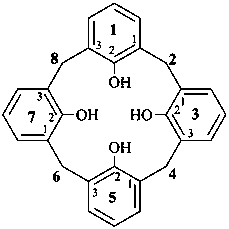
1,3,5,7(1,3)-tetrabenzenacyclooctaphane-12,32,52,72-tetrol (PIN)
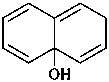
(1) naphthalen-4a(2H)-ol (PIN)
2,4a-dihydronaphthalen-4a-ol
(see P-58.2)
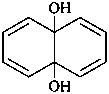
(1) naphthalene-4a,8a-diol (PIN)
4a,8a-dihydronaphthalene-4a,8a-diol
(see P-58.2)
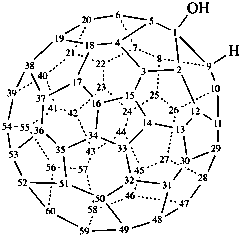
(C60-Ih)[5,6]fulleren-1(9H)-ol (PIN)
1,9-dihydro(C60-Ih)[5,6]fulleren-1-ol
(see P-58.2)
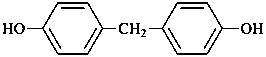
(3) 4,4′-methylenediphenol (PIN)
(1) 4-[(4-hydroxyphenyl)methyl]phenol
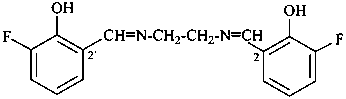
(3) 2,2′-[ethane-1,2-diylbis(azanylylidenemethanylylidene)]bis(6-fluorophenol)(PIN)
(1) 2-fluoro-6-{[(2-{[(3-fluoro-2-hydroxyphenyl)methylidene]amino}ethyl)imino]methyl}phenol
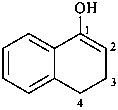
(1) 3,4-dihydronaphthalen-1-ol (PIN)
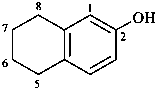
(1) 5,6,7,8-tetrahydronaphthalen-2-ol (PIN)
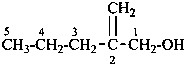
(1) 2-methylidenepentan-1-ol (PIN)
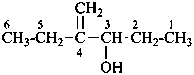
(1) 4-methylidenehexan-3-ol (PIN)
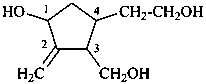
(1) 4-(2-hydroxyethyl)-3-(hydroxymethyl)-2-methylidenecyclopentan-1-ol (PIN)
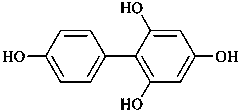
(1) [1,1′-biphenyl]-2,4,4′,6-tetrol (PIN)
biphenyl-2,4,4′,6-tetrol
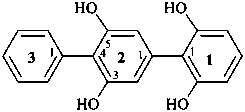
(1) [11,21:24,31-terphenyl]-12,16,23,25-tetrol (PIN)
(in the PIN brackets enclose the name of an assembly requiring
locants when suffixes are present; for numbering, see P-28.3)
[1,1′:4′,1′′-terphenyl]-2,3′,5′,6-tetrol (see P-28.3)
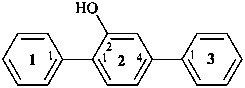
[11,21:24,31-terphenyl]-22-ol (PIN; see P-28.3]
[1,1′:4′,1′′-terphenyl]-2′-ol (see P-28.3)
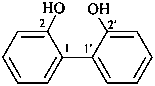
(1) [1,1′-biphenyl]-2,2′-diol (PIN)
2,2′-biphenol
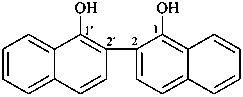
[2,2′-binaphthalene]-1,1′-diol (PIN)
2,2′-bi-1-naphthol
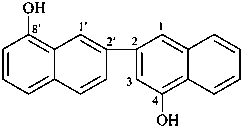
(1) [2,2′-binaphthalene]-4,8′-diol (PIN)
3,7′-bi-1-naphthol
When the hydroxy group is attached to an atom other than carbon, hydroxy compounds belong to a compound class called heterols. They are classified as hydroxy compounds and named using the suffix ‘ol’, unless they are classified as acids and denoted by a retained name. Names formed using a suffix are preferred to those formed by means of the prefix ‘hydroxy’.
Examples:
(CH3-CH2)2AlOH
diethylalumanol
diethyl(hydroxy)alumane
(alumane is a preselected name; see P-12.2)
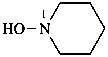
piperidin-1-ol (PIN)
1-hydroxypiperidine
N-hydroxypiperidine
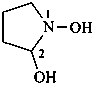
pyrrolidine-1,2-diol (PIN)
1-hydroxypyrrolidin-2-ol
N-hydroxypyrrolidin-2-ol
P(OH)3
phosphorous acid (retained preselected name)
(not phosphanetriol)
H2As-OH
arsinous acid (retained preselected name)
(not arsanol)
Hydroxy groups are indicated by the prefix ‘hydroxy’ when:
(1) a group having priority for citation as the principal characteristic group is present; orExamples:(2) a hydroxy group cannot be denoted by a suffix.
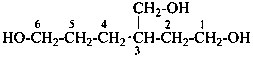
(2) 3-(hydroxymethyl)hexane-1,6-diol (PIN)
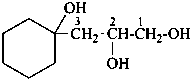
(2) 3-(1-hydroxycyclohexyl)propane-1,2-diol (PIN)
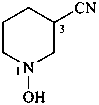
(1) 1-hydroxypiperidine-3-carbonitrile (PIN)
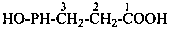
(1) 3-(hydroxyphosphanyl)propanoic acid (PIN)
Sulfur, selenium, and tellurium analogues of hydroxy compounds are named substitutively using the suffixes ‘thiol’, ‘selenol’, and ‘tellurol’, and the prefixes ‘sulfanyl’, ‘selanyl’, and ‘tellanyl’, respectively; the presence of several of the same kind of ‘ol’ characteristic groups is denoted by the numerical multiplying prefixes ‘di’, ‘tri’, etc. The prefixes ‘mercapto’ (–SH), and ‘hydroseleno’ or selenyl (–SeH), etc. are no longer recommended.
Functional class nomenclature is not used.
Names of assemblies of identical units are formed by methods described in P-15.3 and P-51.3. Names for divalent prefixes are described in P-63.2.5. Multiplicative names are preferred to substitutive names when all conditions for their formation are fulfilled (P-51.1.5).
The seniority order of sulfur, selenium, and tellurium analogues of hydroxy compounds is: O > S > Se > Te.
Examples:
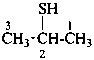
CH3-CH2-SeH
ethaneselenol (PIN)
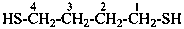
butane-1,4-dithiol (PIN)
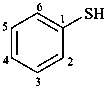
benzenethiol (PIN)
(not thiophenol)
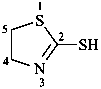
4,5-dihydro-1,3-thiazole-2-thiol (PIN)
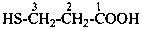
3-sulfanylpropanoic acid (PIN)
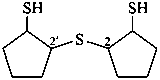
2,2′-sulfanediyldi(cyclopentane-1-thiol) (PIN)
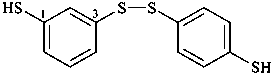
3-[(4-sulfanylphenyl)disulfanyl]benzene-1-thiol (PIN)
3,4′-disulfanediyldi(benzene-1-thiol)
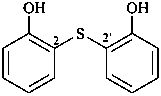
2,2′-sulfanediyldiphenol (PIN)
2-[(2-hydroxyphenyl)sulfanyl]phenol
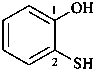
2-sulfanylphenol (PIN)
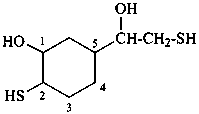
5-(1-hydroxy-2-sulfanylethyl)-2-sulfanylcyclohexan-1-ol (PIN)
(ring preferred to chain, see P-52.2.8)
1-(3-hydroxy-4-sulfanylcyclohexyl)-2-sulfanylethan-1-ol
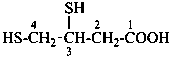
3,4-bis(sulfanyl)butanoic acid (PIN)
P-63.2.1 Definitions and general methodologyP-63.2.1 Definitions and general methodology
P-63.2.2 Names of substituent groups R′-O–, R′-S–, R′-Se–, and R′-Te–
P-63.2.3 Retained names of ethers
P-63.2.4 Systematic names of ethers
P-63.2.5 Names of chalcogen analogues of ethers: i.e., sulfides, selenides, and tellurides
Ethers have the general formula R-O-R′, in which R = R′ or R ≠ R′; R and R′ can be any substituent group, aliphatic or cyclic, organyl (the free valence attached to a carbon atom) or organoheteryl (the free valence attached to an atom other than carbon), derived from the parent hydrides described in P-29.
Examples:
(CH3)3Si-O-CH3
H3Ge-O-GeH3
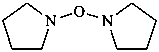
Names for ethers and their chalcogen analogues are formed by various methods in accordance with the principles of substitutive nomenclature, multiplicative nomenclature, skeletal replacement (‘a’) nomenclature, phane nomenclature, and functional class nomenclature. However, some ethers and chalcogen analogues are classified as parent hydrides and named as such, for example H3Ge-O-GeH3, digermoxane, and similar compounds described in Section P-21.2.3.1. These compounds are thus not named by the methods described in this Section, because their names are subject to selection rules with regard to heteroatom content.
In substitutive nomenclature, when R is different from R′, RH is chosen as parent hydride and R′-O– is cited as a substituent to it. Names of these substituent groups are described in Section P-63.2.2. Functional class nomenclature uses substituent group names for R and R′.
P-63.2.2 Names of substituent groups R′-O–, R′-S–, R′-Se–, and R′-Te–
P-63.2.2.1 Systematic names
P-63.2.2.1.1 Substituent prefix names for R′-O– groups are formed by concatenation, i.e., by adding the prefix ‘oxy’ to the substituent prefix name for the group R′. These compound prefixes require the numerical multiplying prefixes ‘bis’, ‘tris’, etc.
Examples:
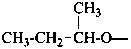
(butan-2-yl)oxy (preferred prefix)
1-methylpropoxy
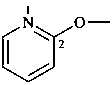
(pyridin-2-yl)oxy (preferred prefix)
2-pyridyloxy
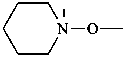
(piperidin-1-yl)oxy (preferred prefix)
piperidinooxy
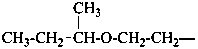
2-[(butan-2-yl)oxy]ethyl (preferred prefix)
H3Si-O—
silyloxy (preselected prefix)
(not siloxy)
Examples:
C6H5-Se–
phenylselanyl (preferred prefix)
phenylseleno
Examples:
–S-CH2-S–
methylenebis(sulfanediyl) (preferred prefix)
–CH2-S-CH2–
sulfanediylbis(methylene) (preferred prefix)
(not sulfanediyldimethylene)
Some contracted names are retained for R-O– substituent groups. They are used both as preferred IUPAC prefixes and in general nomenclature; they are fully substitutable (with the exception of tert-butoxy) and are considered as simple prefixes requiring the numerical prefixes ‘di’, ‘tri’, etc. They are:
CH3-CH2-O–
ethoxy (preferred prefix)
CH3-[CH2]2-O–
propoxy (preferred prefix)
CH3-[CH2]3-O–
butoxy (preferred prefix)
C6H5-O–
phenoxy (preferred prefix)
(CH3)3C-O–
tert-butoxy (preferred prefix)
(no substitution)
(CH3)2CH-CH2-O–
2-methylpropoxy (preferred prefix)
(not isobutoxy)
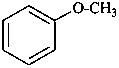
Anisole, C6H5-O-CH3, is the only name in the class of ethers which is retained both as a preferred IUPAC name and for use in general nomenclature. For preferred IUPAC names, no substitution is allowed; for general nomenclature substitution is allowed on the ring and on the side chain under certain conditions (see P-34.1.1.4).
Examples:
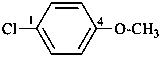
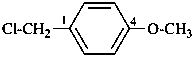
1-(chloromethyl)-4-methoxybenzene (PIN;
no substitution on anisole for PINs)
4-(chloromethyl)anisole
4-methoxybenzyl chloride
(substitution rules for benzyl see P-29.6.2.1)
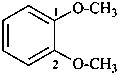
1,2-dimethoxybenzene (PIN;
no substitution on anisole for PINs)
2-methoxyanisole
(see P-34.1.1.4 and P-15.1.8.2 for substitution rules for anisole)
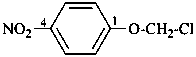
1-(chloromethoxy)-4-nitrobenzene (PIN;
no substitution on anisole for PINs)
α-chloro-4-nitroanisole
(see P-34.1.1.4 and P-15.1.8.2 for substitution rules for anisole)
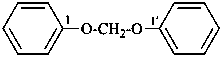
1,1′-[methylenebis(oxy)]dibenzene (PIN)
α-phenoxyanisole
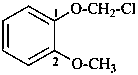
1-(chloromethoxy)-2-methoxybenzene (PIN;
no substitution on anisole for PINs)
α-chloro-2-methoxyanisole
[not 2-(chloromethoxy)anisole]
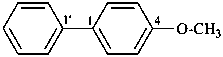
4-methoxy-1,1′-biphenyl (PIN)
(not 4-phenylanisole;
no substitution on anisole for PINs)
[not 1-methoxy-4-phenylbenzene;
the biphenyl ring system is senior to a single benzene ring]
Ethers having the general structure R-O-R′ (R=R′, or R≠R′) have the class name ‘ether’ and are named by one of the five following methods:
(1) substitutively by prefixing the name of the R′-O– group to that of the parent hydride RH;Functional class names based on the class name ‘oxide’ are not recommended.(2) by functional class nomenclature, using the term ‘ether’ and, when the groups are different, citing the two substituent groups in alphanumerical order;
(3) by multiplicative nomenclature, when R and R′ are cyclic components;
(4) by skeletal replacement (‘a’) nomenclature;
(5) by phane nomenclature.
P-63.2.4.1 Names of ethers, when R and R′ are both aliphatic groups or when one is cyclic, are formed by method (1), (2), or (4). Method (1) or (4) leads to preferred IUPAC names.
Examples:
CH3-CH2-O-CH3
(1) methoxyethane (PIN)
(2) ethyl methyl ether
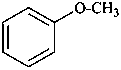
(1) anisole (PIN; retained name)
methoxybenzene
(2) methyl phenyl ether
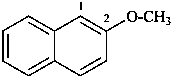
(1) 2-methoxynaphthalene (PIN)
(2) methyl naphthalen-2-yl ether
methyl 2-naphthyl ether
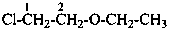
(1) 1-chloro-2-ethoxyethane (PIN)
(2) 2-chloroethyl ethyl ether
(not 2-chloroethyl ethyl oxide)
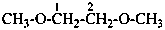
(1) 1,2-dimethoxyethane (PIN)
(2) ethane-1,2-diyl dimethyl ether
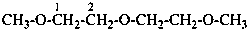
(1) 1-methoxy-2-(2-methoxyethoxy)ethane (PIN)
Example:
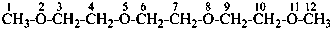
When method (1), substitutive nomenclature, is used, the senior ring or ring system must be chosen as the parent hydride (see P-44).
Examples:
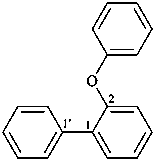
(1) 2-phenoxy-1,1′-biphenyl (PIN)
(2) biphenyl-2-yl phenyl ether
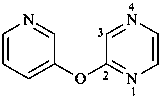
(1) 2-[(pyridin-3-yl)oxy]pyrazine (PIN)
(2) pyrazin-2-yl 3-pyridyl ether
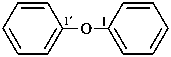
(3) 1,1′-oxydibenzene (PIN)
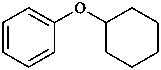
(1) phenoxybenzene
(2) diphenyl ether
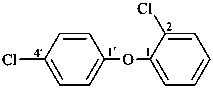
(1) 1-chloro-2-(4-chlorophenoxy)benzene (PIN)
(3) 2,4′-dichloro-1,1′-oxydibenzene (numbering shown)
(2) 2-chlorophenyl 4-chlorophenyl ether
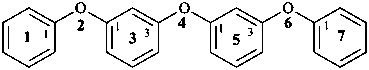
(5) 2,4,6-trioxa-1,7(1),3,5(1,3)-tetrabenzenaheptaphane (PIN)
(3) 1,1′-oxybis(3-phenoxybenzene)
General methodology
Sulfides, R-S-R′, selenides R-Se-R′, and tellurides R-Te-R′, are named by the following methods:
(1) by prefixing the names of the substituent groups R′-S–, R′-Se–, or R′-Te–, i.e., R′-sulfanyl, R′-selanyl, and R′-tellanyl, respectively, to that of the parent hydride, RH; the prefixes ‘R′-thio’, ‘R′-seleno’ and ‘R′-telluro’ may be used in general nomenclature. The prefixes R′-sulfanyl, R′-selanyl, and R′-tellanyl are compulsory prefixes and can be attached to any atom of any parent hydride;Names formed by substituting the parent hydrides oxidane, sulfane, selane, and tellane, H2O, H2S, H2Se, and H2Te, respectively, by the appropriate substituent groups are not recommended.(2) by functional class nomenclature using the terms sulfide, selenide, and telluride for –S–, –Se–, and –Te–, respectively;
(3) by multiplicative nomenclature in the case of cyclic parent hydrides, using the prefixes sulfanediyl, –S– (not thio); selanediyl –Se– (not seleno); and tellanediyl –Te– (not telluro), respectively;
(4) by skeletal replacement (‘a’) nomenclature;
(5) by phane nomenclature;
Names formed by functional replacement nomenclature of the retained name anisole are no longer recommended. Class names such as thiooxide are not recommended.
Method (1), substitutive nomenclature, gives preferred IUPAC names; method (3), (4), or (5) generates preferred IUPAC names when the conditions for their use are satisfied.
Examples:
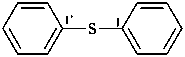
(3) 1,1′-sulfanediyldibenzene (PIN)
(not 1,1′-thiodibenzene)
(1) (phenylsulfanyl)benzene
(phenylthio)benzene
(2) diphenyl sulfide
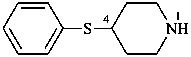
(1) 4-(phenylsulfanyl)piperidine (PIN)
4-(phenylthio)piperidine
(2) phenyl piperidin-4-yl sulfide
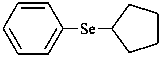
(1) (cyclopentylselanyl)benzene (PIN)
(cyclopentylseleno)benzene
(2) cyclopentyl phenyl selenide
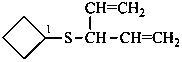
(1) [(penta-1,4-dien-3-yl)sulfanyl]cyclobutane (PIN)
[(penta-1,4-dien-3-yl)thio]cyclobutane
(ring preferred to chain, see P-52.2.8)
(2) cyclobutyl penta-1,4-dien-3-yl sulfide
cyclobutyl 1-ethenylprop-2-en-1-yl sulfide
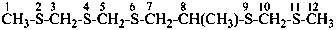
(4) 8-methyl-2,4,6,9,11-pentathiadodecane (PIN; skeletal replacement name)
(1) 2-{[(methylsulfanyl)methyl]sulfanyl}-1-[({[(methylsulfanyl)methyl]sulfanyl}methyl)sulfanyl]propane
2-{[(methylthio)methyl]thio}-1-[({[(methylthio)methyl]thio}methyl)thio]propane
(substitutive names)
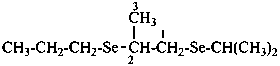
(1) 1-[(propan-2-yl)selanyl]-2-(propylselanyl)propane (PIN)
1-[(propan-2-yl)seleno]-2-(propylseleno)propane
(not 2,5-dimethyl-3,6-diselenanonane;
skeletal replacement (‘a’) nomenclature requires four heterounits, see P-51.4)
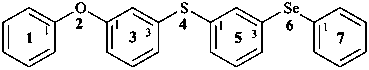
(5) 2-oxa-4-thia-6-selena-1,7(1),3,5(1,3)-tetrabenzenaheptaphane (PIN; phane name)
1-phenoxy-3-{[3-(phenylselanyl)phenyl]sulfanyl}benzene (substitutive name)
not 1-[(3-phenoxyphenyl)sulfanyl]-3-(phenylselanyl)benzene (substitutive name)
(the first substitutive name is correct because phenoxy-phenylselanyl is lower alphabetically than phenoxyphenyl-sulfanyl)
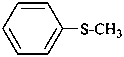
(1) (methylsulfanyl)benzene (PIN)
(not thioanisole)
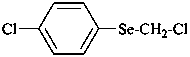
(1) 1-chloro-4-[(chloromethyl)selanyl]benzene (PIN)
(not α,4-dichloroselenoanisole)
P-63.3.1 Peroxides, disulfides, diselenides, and ditellurides
Compounds with the general structures R-OO-R′, R-SS-R′, R-SeSe-R′, and R-TeTe-R′ are named in the following ways:
(1) substitutively, by combining the prefix name for R′ additively with ‘peroxy’ giving the prefixes ‘R′-peroxy’ (not R′-dioxy), ‘R′-disulfanyl’, ‘R′-diselanyl’ or ‘R′-ditellanyl’ attached to the name of the parent hydride corresponding to R;Names formed by substituting the parent hydrides dioxidane, disulfane, diselane, and ditellane HOOH, HSSH, HSeSeH, and HTeTeH, respectively, by the appropriate substituent groups are not recommended.(2) by functional class nomenclature by citing the names of the groups R and R′, in alphanumerical order if two different groups are present, and the class name, peroxide, disulfide, diselenide, or ditelluride, respectively, as a separate word (class names such as dithioperoxide are not recommended);
(3) by multiplicative nomenclature, using the preferred prefixes disulfanediyl, –SS–, diselanediyl, –SeSe–, and ditellanediyl, –TeTe–, respectively; dithio, diseleno, and ditelluro may be used in general nomenclature.
(4) by skeletal replacement (‘a’) nomenclature;
(5) by phane nomenclature.
Method (1), substitutive nomenclature, gives preferred IUPAC names; methods (3), (4), or (5) generate preferred IUPAC names when the conditions for their use are satisfied.
Examples:
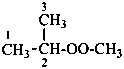
(1) 2-(methylperoxy)propane (PIN)
(2) isopropyl methyl peroxide
methyl 1-methylethyl peroxide
CH3-SS-CH3
(1) (methyldisulfanyl)methane (PIN)
(2) dimethyl disulfide
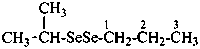
(1) 1-[(propan-2-yl)diselanyl]propane (PIN)
(2) isopropyl propyl diselenide
1-methylethyl propyl diselenide
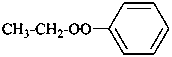
(1) (ethylperoxy)benzene (PIN)
(2) ethyl phenyl peroxide
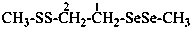
(1) 1-(methyldiselanyl)-2-(methyldisulfanyl)ethane (PIN)
1-(methyldiseleno)-2-(methyldithio)ethane
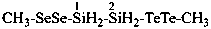
(1) 1-(methyldiselanyl)-2-(methylditellanyl)disilane (PIN)
(disilane is a preselected name, see P-12.2)
1-(methyldiseleno)-2-(methylditelluro)disilane
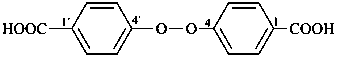
(3) 4,4′-peroxydibenzoic acid (PIN)
4-[(4-carboxyphenyl)peroxy]benzoic acid
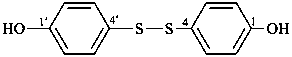
(3) 4,4′-disulfanediyldiphenol (PIN)
4,4′-dithiodiphenol
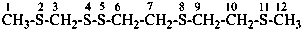
(4) 2,4,5,8,11-pentathiadodecane (PIN)
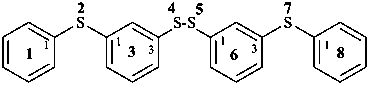
(5) 2,4,5,7-tetrathia-1,8(1),3,6(1,3)-tetrabenzenaoctaphane (PIN)
Mixed chalcogen structures such as R-XY-R′ or R-YX-R′ in which X and Y are O, S, Se, or Te atoms are named by three methods:
(1) by prefixing the names of the substituent groups R′-O–, R′-S–, R′-Se–, or R′-Te–, i.e., R′-oxy, R′-sulfanyl, R′-selanyl, and R′-tellanyl, respectively, to ‘oxy’, ‘sulfanyl’, ‘selanyl’, or ‘tellanyl’ and then to the appropriate parent hydride name. The prefixes R′-sulfanyl, R′-selanyl, and R′-tellanyl are compulsory prefixes and can be attached to any atom of any parent hydride; multiplicative nomenclature is used when the conditions for its use are fulfilled;Methods (1) and (3) lead to preferred IUPAC names.(2) by citing the prefix names of the groups R and R′, in alphanumerical order, followed by an appropriate class name ‘thioperoxide’, ‘diselenoperoxide’, ‘selenothioperoxide’, etc. Each prefix R and R′ is preceded by a capital italicized letter locant, as appropriate;
(3) by skeletal replacement (‘a’) nomenclature or phane nomenclature, when the conditions for its use are fulfilled.
Examples:
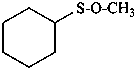
(1) (methoxysulfanyl)cyclohexane (PIN)
(2) S-cyclohexyl O-methyl thioperoxide
(not methyl cyclohexanesulfenate)
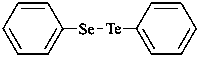
(1) [(phenylselanyl)tellanyl]benzene (PIN)
{not [(phenyltellanyl)selanyl]benzene;
phenylselan... is lower alphabetically than phenyltellan... (see P-14.5)}
(2) diphenyl selenotelluroperoxide
(not diphenyl telluroselenoperoxide;
not phenyl benzenetelluroselenate)
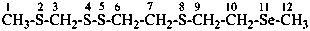
(3) 2,4,5,8-tetrathia-11-selenadodecane (PIN)
[not (methylsufanyl)methyl 2-{[2-(methylselanyl)ethyl]sulfanyl}ethanesufenothioate]
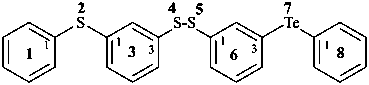
(2) 2,4,5-trithia-7-tellura-1,8(1),3,6(1,3)-tetrabenzenaoctaphane(PIN)
[not 3-(phenylsulfanyl)phenyl 3-(phenyltellanyl)benzenesulfenothioate]
P-63.4.1 Hydroperoxides
The suffix ‘peroxol’ is now introduced to name the group –OOH, formerly named by functional class nomenclature as ‘hydroperoxide’. Chalcogen analogues are names by suffixes such as ‘thioperoxol’, ‘dithioperoxol’, ‘selenoperoxol’, and ‘selenothioperoxol’.
Compounds with the general structure R-OOH are called generically ‘hydroperoxides’. The class name ‘peroxols’ could be more appropriate. They are named in two ways when the –OOH group is the principal characteristic group.
(1) substitutively using the suffix ‘peroxol’;The prefix ‘peroxy’, not ‘dioxy’, is retained for the group –OO– (see P-63.3.1). The prefix ‘hydroperoxy’ is formed by concatenation to describe the group –OOH as a substituent in the presence of a characteristic group having priority for citation as a suffix. Method (1) leads to preferred IUPAC names.(2) by functional class nomenclature using the class name ‘hydroperoxide’.
Examples:
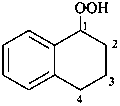
2-hydroperoxy-1-phenylethan-1-one (PIN)
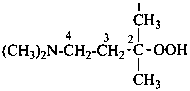
(1) 4-(dimethylamino)-2-methylbutane-2-peroxol (PIN)
(2) 4-(dimethylamino)-2-methylbutan-2-yl hydroperoxide
3-(dimethylamino)-1,1-dimethylpropyl hydroperoxide
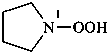
(1) pyrrolidine-1-peroxol (PIN)
(2) pyrrolidin-1-yl hydroperoxide
P-63.4.2.1 Compounds having the general structure R-SOH or R-OSH are called generically ‘thioperoxols’ or ‘thiohydroperoxides’. Similarly, compounds R-SeOH or R-OSeH and R-TeOH or R-OTeH, are called ‘selenoperoxols’ or ‘selenohydroperoxides’, and ‘telluroperoxols’ or ‘tellurohydroperoxides’, respectively. When representing the principal function, they are named by two methods.
(1) by substitutive nomenclature and the appropriate suffix listed in Table 6.1 formed by functional replacement, to denote a principal function;(2) by functional class nomenclature using the name of the class ‘thiohydroperoxide’, ‘selenohydroperoxide’ or ‘tellurohydroperoxide’. When required, the prefixes, ‘thio’, ‘seleno’ and ‘telluro’ are placed in alphabetical order, for example, ‘selenothiohydroperoxide’, etc., and the locants O, S, Se, or Te are used to designate the bonding of the R– group; when two atoms of the same element are present the class name ‘disulfide’, ‘diselenide’, or ‘ditelluride’ is used.
Compounds of the type R-SOH, R-SeOH and R-TeOH and their chalcogen analogues were previously named sulfenic, selenenic and tellurenic acids, using the suffixes ‘sulfenic acid’, ‘selenenic acid’, and ‘tellurenic acid’, respectively.
Method (1) generates preferred IUPAC names.
| Table 6.1 Suffixes denoting peroxols (hydroperoxides) modified by functional replacement nomenclature (in decreasing order of seniority as the principal group) | ||||
| –S-OH | -SO-thioperoxol | –Se-SH | -SeS-selenothioperoxol | |
| –Se-OH | -SeO-selenoperoxol | –Te-SH | -TeS-tellurothioperoxol | |
| –Te-OH | -TeO-telluroperoxol | –S-SeH | -SSe-selenothioperoxol | |
| –O-SH | -OS-thioperoxol | –S-TeH | -STe-tellurothioperoxol | |
| –O-SeH | -OSe-selenoperoxol | –Se-SeH | -diselenoperoxol | |
| –O-TeH | -OTe-telluroperoxol | –Te-SeH | -TeSe-selenotelluroperoxol | |
| –S-SH | -dithioperoxol | –Se-TeH | -SeTe-selenotelluroperoxol | |
| –Te-TeH | -ditelluroperoxol | |||
Examples:
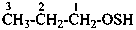
(1) propane-1-OS-thioperoxol (PIN)
(2) O-propyl thiohydroperoxide
CH3-CH2-SSH
(1) ethanedithioperoxol (PIN)
(2) ethyl hydrodisulfide
ethyl dithiohydroperoxide
CH3-SSeH
(1) methane-SSe-selenothioperoxol (PIN)
(2) S-methyl selenothiohydroperoxide
(1) by using prefixes such as ‘hydroperoxy’, –OOH; ‘disulfanyl’, –SSH, or by combining simple prefixes, ‘hydroxy’ –OH; ‘oxy-’, –O–; ‘sulfanyl’, –SH; etc.;Method (1) leads to preferred IUPAC names.(2) by using prefixes such as dithiohydroperoxy, –SSH; SO-thiohydroperoxy, –OSH; SeS-selenothiohydroperoxy, –SSeH; etc.
Examples:
HSS-CH2-COOH
(1) disulfanylacetic acid (PIN)
(2) (dithiohydroperoxy)acetic acid
(1) 3,4-bis(disulfanyl)benzamide (PIN)
(2) 3,4-bis(dithiohydroperoxy)benzamide
HS-O-CH2-CH2-CN
(1) 3-(sulfanyloxy)propanenitrile (PIN)
(2) 3-(SO-thiohydroperoxy)propanenitrile
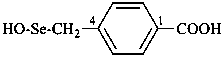
(1) 4-[(hydroxyselanyl)methyl]benzoic acid (PIN)
(2) 4-[(OSe-selenohydroperoxy)methyl]benzoic acid
Cyclic ethers, sulfides, selenides and tellurides are heterocycles named by the following methods:
(1) by using preferred retained names described in P-22.2.1 (chosen first);Names of heterocyclic compounds are preferred IUPAC names.(2) for monocycles, by the extended Hantzsch-Widman system (see P-22.2.2) or by skeletal replacement (‘a’) nomenclature for cycles having more than ten members (see P-22.2.3);
(3) by bridged fused nomenclature (see P-25.4);
(4) by using detachable prefixes ‘epoxy’, ‘epithio’, ‘episeleno’, or ‘epitelluro’ in substitutive nomenclature; these are used primarily in the nomenclature of natural products (see P-101.5.2); however they can also be used in general nomenclature.
(5) by using additive names formed by the addition of the terms ‘oxide’, ‘sulfide’, ‘selenide’, or ‘telluride’ to the name of an unsaturated compound.
Examples:
(1) tellurophene (PIN)
(2) oxolane (PIN)
(1) tetrahydrofuran
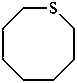
(2) thiocane (PIN)
(2) oxacyclotridecane (PIN)
(3) 1,4-dihydro-1,4-sulfanonaphthalene (PIN)
(2) 2-ethyl-2-methyloxirane (PIN)
(4) 1,2-epoxy-2-methylbutane
(2) oxirane (PIN)
(5) ethene oxide
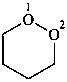
(2) 1,2-dioxane (PIN)
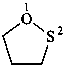
(2) 1,2-oxathiolane (PIN)
Compounds with the general structures R-SO-R′ and R-SO2-R′ are called generically ‘sulfoxides’ and ‘sulfones’, respectively, when R and R′ are hydrocarbyl groups. They are named in three ways as follows:
(1) substitutively, by prefixing the name of the acyl group R′-SO– or R′-SO2– to the name of the parent hydride corresponding to R as described in P-65.3.2.2.2;Methods (1) and (3) generate preferred names.(2) by functional class nomenclature, using the class names ‘sulfoxide’ and ‘sulfone’, respectively;
(3) by multiplicative nomenclature, except where R and R′ are alkyl groups, when the conditions for its use are satisfied.
Selenium and tellurium analogues are named in the same way using acyl groups derived from the appropriate seleninic, selenonic, tellurinic, and telluronic acids, and the class names ‘selenoxide’, ‘selenone’, ‘telluroxide’, ‘tellurone’. Prefixes such as ‘alkylsulfinyl’ or ‘arylsulfonyl’ may be used in general nomenclature.
Di- and polysulfones are described in P-68.4.3.2
Examples:
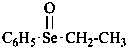
(1) (ethaneseleninyl)benzene (PIN)
(ethylseleninyl)benzene
(2) ethyl phenyl selenoxide
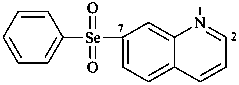
(1) 7-(benzeneselenonyl)quinoline (PIN)
7-(phenylselenonyl)quinoline
(2) phenyl quinolin-7-yl selenone
phenyl 7-quinolyl selenone
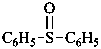
(3) 1,1′-sulfinyldibenzene (PIN)
(2) diphenyl sulfoxide
(1) (benzenesulfinyl)benzene
(phenylsulfinyl)benzene
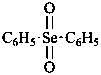
(3) 1,1′-selenonyldibenzene (PIN)
(2) diphenyl selenone
(1) (benzeneselenonyl)benzene
(phenylselenonyl)benzene
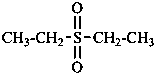
(1) (ethanesulfonyl)ethane (PIN)
(ethylsulfonyl)ethane
(2) diethyl sulfone
Note: Multiplication of acyclic hydrocarbons is not permitted
In the order of seniority of classes, hydroxy compounds and hydroperoxides are ranked in descending order after aldehydes and ketones, but before amines and imines. Chalcogen analogues are ranked after each of these classes, according to the number of O, S, Se and Te atoms. In descending order, they are as follows.
(1) hydroxy compounds –OH, then their chalcogen analogues, –SH > –SeH > –TeHThere is no seniority order between phenols and hydroxy compounds. The choice for parent hydride is decided by the maximum number of hydroxy groups cited as suffixes; and a ring is preferred to a chain when there is a choice (see P-52.2.8).(2) hydroperoxides –OOH, then their chalcogen analogues, –SOH > –SeOH > –TeOH, etc. (see Table 6.1)
(3) amines > imines
(4) ethers, –O–, then their chalcogen analogues, –S– > –Se– > –Te–
(5) peroxides –OO–, then their chalcogen analogues, –OS– > –OSe– > –OTe–, > –SS– > –SSe– > –STe– > –SeSe– > –SeTe– > –TeTe–.
Examples:
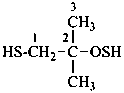
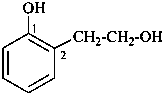
2-(2-hydroxyethyl)phenol (PIN)
2-(2-hydroxyphenyl)ethan-1-ol
(the ring is senior to the chain in the preferred name, see P-52.2.8)
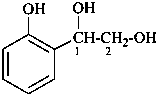
1-(2-hydroxyphenyl)ethane-1,2-diol (PIN)
[not 2-(1,2-dihydroxyethyl)phenol;
two principal groups are senior to one]
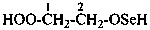
2-(selanyloxy)ethane-1-peroxol (PIN)
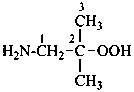
1-amino-2-methylpropane-2-peroxol (PIN)
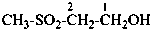
2-(methanesulfonyl)ethan-1-ol (PIN)
2-(methylsulfonyl)ethan-1-ol
2-[(2-hydroperoxy-1-hydroxycyclohexyl)peroxy]cyclohexan-1-one (PIN)
(a ketone is senior to alcohols and peroxols)
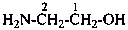
2-aminoethan-1-ol (PIN)
(not ethanolamine)
1-(ethylperoxy)-2-methoxyethane (PIN)
(not [(2-methoxyethyl)peroxy]ethane;
the ‘PIN‘ has more substituents)
1-methoxy-3-(methylsulfanyl)propane (PIN)
1-methoxy-3-(methylthio)propane
1-(methyldisulfanyl)-1-(methylsulfanyl)pent-1-ene (PIN)
{not methyl 1-(methylsulfanyl)pent-1-en-1-yl disulfide;
nor methyl[1-(methylsulfanyl)pent-1-en-1-yl]disulfane; see P-41}
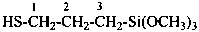
3-(trimethoxysilyl)propane-1-thiol (PIN)
[not trimethoxy(3-sulfanylpropyl)silane;
the suffix, ‘thiol’, has precedence over silane)
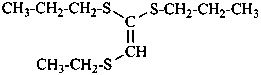
1-{[2-(ethylsulfanyl)-1-(propylsulfanyl)ethen-1-yl]sulfanyl}propane (PIN)
(multiplication of acyclic hydrocarbons is not permitted)
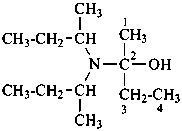
2-[di(butan-2-yl)amino]butan-2-ol (PIN)
2-[bis(1-methylpropyl)amino]butan-2-ol.
[not 2-(di-sec-butylamino)butan-2-ol]
3-[amino(methyl)silyl]-3-[(aminomethyl)silyl]cyclopentan-1-ol (PIN)
{not 3-[(aminomethyl)silyl]-3-[amino(methyl)silyl]cyclopentan-1-ol;
alphanumerical characters are identical;
at the fourth character of the name, the letter ‘a’ is preferred to an open parenthesis (see P-14.6)}
P-63.8.1 Neutral salts of hydroxy compounds and their chalcogen analogues and peroxy compounds are named by citing the cation(s) followed by the name of the anion as a separate word.
According to P-72.2.2.2.2, an anion formed by subtracting a hydron from the chalcogen atom of a hydroxy compound or a chalcogen analogue, or a peroxy compound, that can be expressed by a suffix such as ‘ol’, ‘thiol’, ‘-peroxol’, etc., is named by using compound suffixes ‘olate’, ‘thiolate’, ‘peroxolate’, etc., formed by addition of the ending ‘ate’ to the suffixes ‘ol’, ‘thiol’, ‘peroxol’, etc. The multiplicative prefixes ‘bis’, ‘tris’, etc. are used before such compound suffixes, to avoid any ambiguity.
The traditional names methoxide, ethoxide, propoxide, butoxide and phenoxide, for CH3-O–, C2H5-O–, C3H7-O–, C4H9-O– and C6H5-O–, are retained as preferred IUPAC names and aminoxide, H2N-O–, as a preselected name, and they may be substituted in the same way as the corresponding alcohols. The traditional name tert-butoxide for (CH3)3C-O– is also retained as a preferred IUPAC name but cannot be substituted. The traditional name isopropoxide for (CH3)2CH-O– is retained for general nomenclature but cannot be substituted.
Examples:
CH3-CH2-CH2-O– Na+
sodium propoxide (PIN)
sodium propan-1-olate
(CH3)2CH-O– K+
potassium propan-2-olate (PIN)
potassium isopropoxide
C6H5-O– Li+
lithium phenoxide (PIN)
lithium phenolate
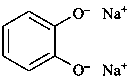
disodium benzene-1,2-bis(olate) (PIN)
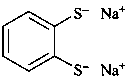
disodium benzene-1,2-bis(thiolate) (PIN)
(C6H5-O–)4 Pb4+
lead tetraphenoxide (PIN)
Example:
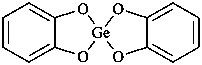
Examples:
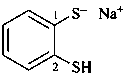
sodium 2-sulfanylbenzene-1-thiolate (PIN)
P-64.0 IntroductionP-64.0 INTRODUCTION
P-64.1 Definitions
P-64.2 Ketones
P-64.3 Pseudoketones
P-64.4 Heterones
P-64.5 Carbonyl groups as prefixes
P-64.6 Chalcogen analogues of ketones, pseudoketones and heterones
P-64.7 Polyfunctional ketones, pseudoketones and heterones
P-64.8 Acyloins
The substitutive nomenclature of ketones is well established. The suffix ‘one’ is used to denote a principal characteristic group, and the prefix ‘oxo’ is used when a characteristic group having seniority is present. The suffix ‘one’ and the prefix ‘oxo’ were indiscriminately used to name some classes of compounds other than ketones. Full systematization based on the strict application of the suffix ‘one’ for denoting the principal characteristic group =O is recommended in this Section.
Traditionally, the nomenclature of ketones was described with that of aldehydes. In these recommendations, the two classes are discussed separately (for aldehydes, see P-66.6), to emphasize the similarities between carboxylic acids and aldehydes with respect to nomenclature. Finally, to avoid fragmentation, the nomenclature of acetals and ketals is discussed with that of aldehydes in Section P-66.6.
Rules on ketones and their chalcogen analogues, discussed as Rules C-311 to C-318 in the 1979 Recommendations (ref. 1) and Rules R-5.6.2 in the 1993 Recommendations (ref. 2) are superseded by the corresponding rules described in this Section, P-64.
P-64.1 DEFINITIONS
P-64.1.1 Ketones are defined classically as compounds in which a carbonyl group is bonded to two carbon atoms: R2CO (neither R may be H) (see ref. 23).
Example:
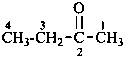
The adjunction of these two new subclasses to the general class of ketones, clarifies the general use of suffixes and prefixes in substitutive nomenclature by always giving precedence to suffixes that designate a principal characteristic group.
P-64.1.2.1 Pseudoketones
Pseudoketones are of two types:
(a) cyclic compounds in which a carbonyl group in a ring is bonded to one or two skeletal heteroatoms; orExamples:(b) compounds in which an acyclic carbonyl group is bonded to one or two acyclic skeletal heteroatoms, except nitrogen, halogen, or pseudohalogen atoms, or to a heteroatom of a ring or ring system. When the heteroatom of the ring is a nitrogen atom the compound has been called an unexpressed or ‘hidden amide’.
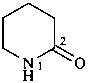
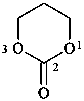
(a) 1,3-dioxan-2-one (PIN)
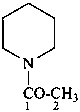
(b) 1-(piperidin-1-yl)ethan-1-one (PIN)
1-acetylpiperidine (a ‘hidden amide’)
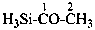
(b) 1-silylethan-1-one (PIN)
acetylsilane
(b) 1-phosphanylpropan-1-one (PIN)
propanoylphosphane
(b) 1-(methoxydisulfanyl)ethan-1-one (PIN)
(see also P-68.4.2)
(b) 1-[(methoxysulfanyl)oxy]propan-1-one (PIN)
(see also P-68.4.2)
Heterones are compounds having an oxygen atom formally doubly bonded to a heteroatom (see P-61.6 and P-64.4; see also P-68). They are named in the same way as ketones except when expressed as compulsory prefixes, such as sulfonyl (see Table 5.1 and P-59.1.9)
Examples:
CH3SiH=O
methylsilanone (PIN)
methyl(oxo)silane
C6H5-P=O
phenylphosphanone (PIN)
oxo(phenyl)phosphane
(not phosphorosobenzene)
P-64.2.1 Retained names
P-64.2.1.1 The name ‘chalcone’ is the only retained name as a preferred IUPAC name and is limited to ring substitution only by characteristic groups lower than ‘ketone’. Chalcone refers only to the trans- or (E)- stereoisomer.
4-[(1E)-3-(2,4-dihydroxyphenyl)-3-oxoprop-1-en-1-yl]benzamide (PIN)
(not 2′,4′-dihydroxychalcone-4-carboxamide)
Examples:
ketene
ethenone (PIN)
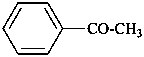
acetophenone
1-phenylethan-1-one (PIN)
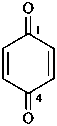
1,4-benzoquinone
cyclohexa-2,5-diene-1,4-dione (PIN)
(not benzoquinone)
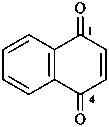
1,4-naphthoquinone
(1,4 isomer shown)
naphthalene-1,4-dione (PIN)
(not naphthoquinone)
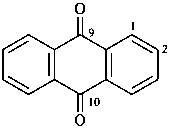
9,10-anthraquinone
anthracene-9,10-dione (PIN)
(not anthraquinone)
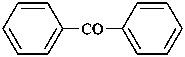
benzophenone
diphenylmethanone (PIN)
(not 1,1′-carbonyldibenzene)
isoquinolin-1(2H)-one (PIN)
[not isoquinolone (1-isomer shown)]
quinolin-2(1H)-one (PIN)
[not quinolone (2-isomer shown)]
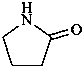
pyrrolidin-2-one (PIN)
[not pyrrolidone (2-isomer shown)]
C6H5-CO-CO-C6H5
diphenylethanedione (PIN)
(not benzil)
CH3-CO-CO-CH3
butane-2,3-dione (PIN)
(not biacetyl)
C6H5-CO-CH2-CH3
1-phenylpropan-1-one (PIN)
(not propiophenone)
P-64.2.2.1 Acyclic ketones
Unsubstituted acyclic ketones are systematically named in two ways:
(1) substitutively, using the suffix ‘one’ and the prefix ‘oxo’; the presence of several ‘one’ characteristic groups is denoted by the numerical multiplying prefixes ‘di’, ‘tri’, etc.; the final letter ‘a’ of a numerical multiplying prefix is elided before the suffix ‘-one’, for example, ‘tetrone’;Method (1) generates preferred IUPAC names.(2) by functional class nomenclature using the class names ‘ketone’, ‘diketone’ etc.; substituent groups are placed, as separate words, in alphanumerical order before the class name.
Examples:
butan-2-one (PIN)
ethyl methyl ketone
(not methyl ethyl ketone;
groups must be cited in alphanumerical order)
heptan-3-one (PIN)
butyl ethyl ketone
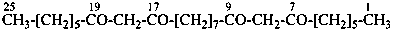
pentacosane-7,9,17,19-tetrone (PIN)
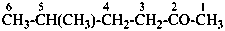
5-methylhexan-2-one (PIN)
methyl 3-methylbutyl ketone
(not isopentyl methyl ketone)
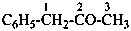
1-phenylpropan-2-one (PIN)
benzyl methyl ketone
1-phenylethan-1-one (PIN)
acetophenone (no substitution)
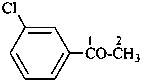
1-(3-chlorophenyl)ethan-1-one (PIN)
(not 3′-chloroacetophenone;
no substitution allowed for acetophenone)
2-bromo-1-(4-chlorophenyl)ethan-1-one (PIN)
(not 4-chlorophenacyl bromide;
not 2-bromo-4′-chloroacetophenone;
no substitution allowed for acetophenone)
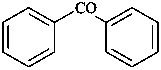
diphenylmethanone (PIN)
benzophenone
diphenyl ketone
di(naphthalen-2-yl)ethanedione (PIN)
di(2-naphthyl)ethanedione
di(2-naphthyl) diketone
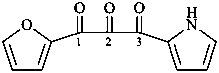
1-(furan-2-yl)-3-(1H-pyrrol-2-yl)propane-1,2,3-trione (PIN)
1-(2-furyl)-3-(pyrrol-2-yl)propanetrione
2-furyl pyrrol-2-yl triketone
Names of cyclic ketones are formed substitutively by using the suffix ‘one’. As the formation of ketones is achieved by the conversion of a methylene, >CH2, group into a >C=O group, the suffix ‘one’ with appropriate locants can be added to the name of parent hydrides having such groups. Methylene groups occur in saturated rings and ring systems and in mancude compounds having indicated hydrogen atoms.
Compounds not having suitably located indicated hydrogen atoms or composed only of =CH– groups, must be hydrogenated in order to create >CH2 groups; when the hydrogenation operation occurs simultaneously with substitution by the >C=O, it is called ‘added indicated hydrogen’ (see P-14.7 and P-58.2.2). The ‘added indicated hydrogen’ method generates preferred IUPAC names.
P-64.2.2.2.1 Alicyclic ketones
Ketones resulting from the substitution of >CH2 groups are named substitutively using the suffix ‘one’ to designate the principal characteristic group.
Examples:
bicyclo[3.2.1]octan-2-one (PIN)
spiro[4.5]decane-1,7-dione (PIN)
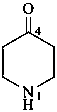
piperidin-4-one (PIN)
Ketones derived from mancude parent hydrides having indicated hydrogen atoms are named by direct substitution of a >CH2 group as indicated in P-64.2.2.2.1. When no indicated hydrogen is present, the methodology of ‘added indicated hydrogen’ is applied (see P-14.7 and P-58.2.2).
Examples:
1H-inden-1-one (PIN)
inden-1-one
naphthalen-1(2H)-one (PIN)
1,2-dihydronaphthalen-1-one
(see P-58.2)
pyrene-1,3,6,8(2H,7H)-tetrone (PIN)
1,2,3,6,7,8-hexahydropyrene-1,3,6,8-tetrone
(see P-58.2)
No retained quinone names are used as preferred IUPAC names. The name 1,4-benzoquinone, and those of naphthoquinones and anthraquinones with locants, are retained for use in general nomenclature with substitution. All other quinones are named systematically using substitutive nomenclature in accordance with P-64.2.2.2.2. Diketones derived from mancude compounds without indicated hydrogen atoms by conversion of two or four =CH– groups into >C=O groups with any rearrangement of double bonds to a quinonoid structure are named systematically (see P-64.2.2.2.2).
Examples:
2-chlorocyclohexa-2,5-diene-1,4-dione (PIN)
2-chloro-1,4-benzoquinone
(not 2-chloro-p-benzoquinone)
naphthalene-1,2-dione (PIN)
1,2-naphthoquinone
2-chloro-3-(pyrrolidin-1-yl)naphthalene-1,4-dione (PIN)
2-chloro-3-(pyrrolidin-1-yl)-1,4-naphthoquinone
anthracene-1,2-dione (PIN)
1,2-anthraquinone
2-methylanthracene-9,10-dione (PIN)
2-methyl-9,10-anthraquinone
quinoline-5,8-dione (PIN)
(not quinoline-5,8-quinone)
chrysene-6,12-dione (PIN)
(not chrysene-6,12-quinone)
acenaphthylene-1,2-dione (PIN)
(not acenaphthoquinone)
When there is a choice for numbering, the starting point and the direction of numbering of a compound are chosen so as to give lowest locants to the structural features (if present) listed in P-14.4. Rule P-52.2.8 is applied when a choice for the principal chain or senior ring system is required.
Examples:
1-selenacyclotridecan-3-one (PIN)
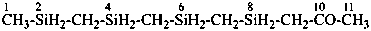
2,4,6,8-tetrasilaundecan-10-one (PIN)
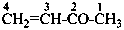
but-3-en-2-one (PIN)
pent-3-yn-2-one (PIN)
pent-1-en-4-yn-3-one (PIN)
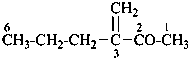
3-methylidenehexan-2-one (PIN)
(11Z)-1,4,7,10(2,5)-tetrafuranacyclododecaphan-11-en-2-one (PIN)
3,4,4a,9,9a,10-hexahydroanthracene-1,2-dione (PIN)
3,4,4a,9,9a,10-hexahydro-1,2-anthraquinone
1,2,3,4,4a,9,9a,10-octahydroanthracene-1,2-dione (see P-58.2)
3,4-dihydronaphthalen-1(2H)-one (PIN)
1,2,3,4-tetrahydronaphthalen-1-one (see P-58.2)
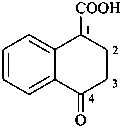
4-oxo-1,2,3,4-tetrahydronaphthalene-1-carboxylic acid (PIN)
5-oxo-1,3,4,5-tetrahydronaphthalene-4a(2H)-carboxylic acid (PIN)
5-oxo-2,5-dihydrofuran-2-carboxylic acid (PIN)
5-oxo-4,5-dihydrofuran-2-carboxylic acid (PIN)
Ketene is the class name for H2C=C=O and its derivatives; the name ketene can be used in general nomenclature to name the unsubstituted structure and derivatives named by compulsory prefixes (see Table 5.1). Other derivatives are named by using the principles for naming ketones.
Examples:
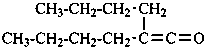
cyclohexylidenemethanone (PIN)
Br2C=C=O
dibromoethenone (PIN)
dibromoketene
Pseudoketones are compounds having a carbonyl group joined to a carbon atom and a heteroatom, –C-CO-X–, or to two heteroatoms, –X-CO-X–, where X ≠ F, Cl, Br, I, pseudohalogen, or acyclic N. These compounds are named substitutively using the suffix ‘one’, in accordance with rules expressed for ketones, when required.
P-64.3.1 Cyclic anhydrides, esters and amides are named as pseudoketones; the resulting names are preferred IUPAC names.
Examples:
azepan-2-one (PIN)
hexano-6-lactam (see P-66.1.5.1)
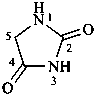
imidazolidine-2,4-dione (PIN)
pyrrolidin-2-one (PIN)
(not 2-pyrrolidone)
quinolin-2(1H)-one (PIN)
(not 1,2-dihydroquinolin-2-one)
isoquinolin-1(2H)-one (PIN)
(not 1,2-dihydroisoquinolin-1-one)
1,2-dihydro-3H-indol-3-one (PIN)
(not 1H-indol-3(2H)-one; see P-58.2)
1,3-diazinane-2,4,6-trione (PIN)
pyrimidine-2,4,6(1H,3H,5H)-trione
1,3,5-triazinane-2,4,6-trione (PIN)
1,3,5-triazine-2,4,6(1H,3H,5H)-trione
2′H,5H-[2,3′-bifuranylidene]-2′,5-dione (PIN)
2′H,4′H-[2,3′-bipyranylidene]-2′,5(6H)-dione (PIN)
2′H,4′H-[2,3′-bipyranylidene]-4′,6(5H)-dione (PIN)
Examples:
1-(3,4-dihydroquinolin-1(2H)-yl)ethan-1-one (PIN)
1-acetyl-1,2,3,4-tetrahydroquinoline
(a hidden amide)
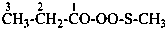
1-[(methylsulfanyl)peroxy]propan-1-one (PIN)
(see also P-68.4.2.4)
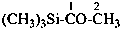
1-(trimethylsilyl)ethan-1-one (PIN)
acetyltri(methyl)silane
Heterones are compounds having an oxygen atom formally doubly bonded to a heteroatom, see P-64.1.2.2; for heterols, see P-63.1.3; for heteroimines, see P-62.3.1.3; see also P-68.3.2.3.1 and P-68.4.3.2; and see also P-68. They are named in the same way as ketones except when they are expressed as compulsory prefixes, such as sulfonyl (see Table 5.1 and P-59.1.9).
P-64.4.1 Acyclic heteronesP-64.4.1 Acyclic heterones are compounds having an oxygen atom doubly bonded to a heteroatom. They may be named in two ways.
P-64.4.2 Thioketone and thioaldehyde oxides
(1) by the suffix ‘one’;Method (1) leads to preferred IUPAC names.(2) by functional class names using the class name ‘oxide’ when the oxygen atom is bonded to a S, Se, Te, P, As, Sb, or Bi atom.
The distinction between ketones, ‘C-CO-C’, and aldehydes, ‘C-CHO’, is not retained for naming compounds having the oxygen atom linked to a heteroatom. Sulfones, sulfoxides, and related chalcogen compounds are exceptions (see P-63.6)
Examples:
(CH3)2Si=O
(1) dimethylsilanone (PIN)
(C6H5)3PO
(1) triphenyl-λ5-phosphanone (PIN, see P-74.2.1.4)
(2) triphenylphosphane oxide
triphenylphosphine oxide
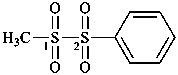
(1) 1-methyl-2-phenyl-1λ6,2λ6-disulfane-1,1,2,2-tetrone (PIN)
(2) methyl phenyl disulfone
Thioketone oxides are named by three methods.
(1) substitutively, as heterones, using the λ-convention and the suffix ‘one’;Method (1) leads to preferred IUPAC names.(2) by functional class nomenclature, using the class names ‘oxide’, and ‘dioxide’, as required.
(3) substitutively, based on the preferred parent structure
Example:
Examples:
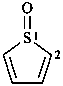
5H-λ6-thianthrene-5,5-dione (PIN)
thianthrene 5,5-dioxide
5,5-dioxo-5H-5λ6-thianthrene
When a carbonyl group is not the principal characteristic group expressed as a suffix, it is denoted by a prefix. The traditional group ‘acetonyl’, for CH3-CO-CH2–, is retained for use in general nomenclature only; the names ‘acetonylidene’ and ‘acetonylidyne’ are not recommended even for general nomenclature. Three types of prefixes are used in the formation of preferred IUPAC names:
(1) the prefix ‘oxo’ when the doubly bonded oxygen atom (ketone, pseudoketone or heterone group) is not in position 1 of a side chain. Lowest possible locants are assigned to suffixes, and then to prefixes;P-64.5.1 Ketones(2) carbonyl groups in position 1 of a side chain, i.e., –CO-R, are described by the appropriate acyl group name (see P-65.4 for names of acyl groups);
(3) the group –CO– is named in substitutive nomenclature as the acyl group ‘carbonyl’; the group =C=O is named in substitutive nomenclature as ‘oxomethylidene’; the substituent group –CHO is named in substitutive nomenclature as the acyl group ‘formyl’.
The prefix ‘oxo’ and/or acyl prefixes are used to denote carbonyl groups when:
(a) all carbonyl or oxo groups cannot be cited as suffixes; orExamples:(b) in the presence of a characteristic group having priority to be cited as suffix.
5-acetylnonane-4,6-dione (PIN)
[not 5-(1-oxoethyl)nonane-4,6-dione]
3-oxobutanoic acid (PIN)
(not acetylacetic acid)
9,10-dioxo-9,10-dihydroanthracene-2-carboxylic acid (PIN)
(not 9,10-anthraquinone-2-carboxylic acid)
4,4′-carbonyldibenzoic acid (PIN; multiplicative name)
4,4′-(oxomethylene)dibenzoic acid
4-(4-carboxybenzoyl)benzoic acid (substitutive name)
P-64.5.2.1 In cyclic pseudoketones, the prefix ‘oxo’ and/or acyl group prefixes are used to denote a carbonyl group:
(a) when all carbonyl groups cannot be cited as suffixes; orAcyl prefixes are used to name pseudoketones (‘hidden amides’) having the structure R-CO-N< where the nitrogen atom is part of a ring or ring system; however, this method is recommended only for general nomenclature. Preferred IUPAC names are formed systematically (see P-64.2.2)(b) in the presence of a characteristic group having priority to be cited as suffix;
Examples:
3-propanoylphosphepan-2-one (PIN)
3-propionylphosphepan-2-one
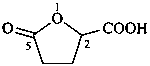
5-oxooxolane-2-carboxylic acid (PIN)
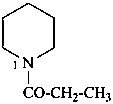
1-(piperidin-1-yl)propan-1-one (PIN)
1-propanoylpiperidine
(a ‘hidden amide’)
1-propionylpiperidine
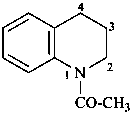
1-(3,4-dihydroquinolin-1(2H)-yl)ethan-1-one (PIN)
1-acetyl-1,2,3,4-tetrahydroquinoline
(a ‘hidden amide’)
Examples:
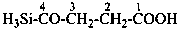
4-oxo-4-silylbutanoic acid (PIN)
3-(silanecarbonyl)propanoic acid
3-(silylcarbonyl)propanoic acid
P-64.6.1 Chalcogen analogues of ketones, pseudoketones and heterones are named by using the following suffixes and prefixes:
=S ‘-thione’ and ‘sulfanylidene’ (preferred to ‘thioxo’)Acyl group prefixes are named by functional replacement of O by S, Se, and Te using infixes (see P-65.1.7). The use of functional replacement prefixes ‘thio’ or ‘seleno’ with retained names is no longer recommended; all preferred IUPAC names are systematically constructed.=Se ‘-selone’ and ‘selanylidene’ (preferred to ‘selenoxo’)
=Te ‘-tellone’ and ‘tellanylidene’ (preferred to ‘telluroxo’)
The use of the prefixes ‘sulfanylidene’, ‘selanylidene’ and ‘tellanylidene’, for =S, =Se, and =Te, respectively, is a change for designating of chalcogen analogs of the ‘oxo’ prefix in preferred IUPAC names. The prefixes ‘thioxo’, ‘selenoxo’, and ‘telluroxo’ derived by functional replacement nomenclature may be used in general nomenclature.
Examples:
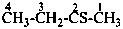
hexane-3-selone (PIN)
pentane-2,4-dithione (PIN)
propane-2-thione (PIN)
(not thioacetone)
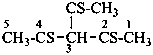
3-(ethanethioyl)pentane-2,4-dithione (PIN)
3-(thioacetyl)pentane-2,4-dithione
3-(1-sulfanylideneethyl)pentane-2,4-dithione
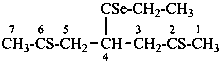
4-(propaneselenoyl)heptane-2,6-dithione (PIN)
4-(1-selanylidenepropyl)heptane-2,6-dithione
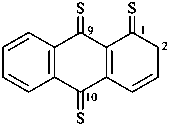
anthracene-1,9,10(2H)-trithione (PIN)
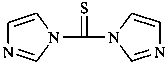
di(1H-imidazol-1-yl)methanethione (PIN)
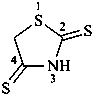
1,3-thiazolidine-2,4-dithione (PIN)
azepane-2-thione (PIN)
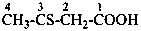
3-sulfanylidenebutanoic acid (PIN)
3-thioxobutanoic acid
4-(3-selanylidenebutyl)benzoic acid (PIN)
4-(3-selenoxobutyl)benzoic acid
The order of seniority of ketonic suffixes is C=O > C=S > C=Se > C=Te. Lowest locants are assigned in accordance with that order.
Examples:
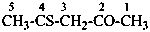
2-sulfanylidene-1,3-thiazolidin-4-one (PIN)
2-thioxo-1,3-thiazolidin-4-one
1,1′-carbonothioyldi(pyridin-2(1H)-one) (PIN)
1,1′-thiocarbonyldi(pyridin-2(1H)-one)
P-64.7.1 Ketones, pseudoketones and heterones, and their chalcogen analogues in the order =O > =S > =Se > =Te, are senior to hydroxy compounds and their chalcogen analogues, amines, and imines in the seniority order of classes. In the presence of a characteristic group having priority to be cited as suffix as described in P-64.5 and P-64.6, they are cited as prefixes (see P-41).
Examples:
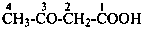
3-oxobutanoic acid (PIN)
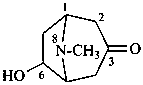
6-hydroxy-8-methyl-8-azabicyclo[3.2.1]octan-3-one
6-hydroxy-8-methyltropan-3-one
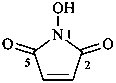
1-hydroxy-1H-pyrrole-2,5-dione (PIN)
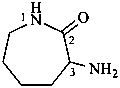
3-aminoazepan-2-one (PIN)
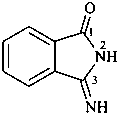
3-imino-2,3-dihydro-1H-isoindol-1-one (PIN)
3-methyl-4-(morpholin-4-yl)-2,2-diphenyl-1-(pyrrolidin-1-yl)butan-1-one (PIN)
1-[4-(3,4-dihydroisoquinoline-2(1H)-carbonyl)piperidin-1-yl]-2,2,3,3,4,4,5,5,6,6,7,7,8,8,8-pentadecafluorooctan-1-one (PIN,
the
locants for the fluoro substituents are required, see P-14.3.4.5)
2,5-dichloro-3,6-dihydroxycyclohexa-2,5-diene-1,4-dione (PIN)
2,5-dichloro-3,6-dihydroxy-1,4-benzoquinone
1,8-dihydroxy-3-methylanthracene-9,10-dione (PIN)
1,8-dihydroxy-3-methyl-9,10-anthraquinone
Examples:
4-(4-oxocyclohexyl)oxolan-2-one (PIN)
[not 4-(2-oxooxolan-4-yl)cyclohexanone;
a heterocyclic ring is senior to a carbocyclic ring, see P-44.2.1)
Example:
α-Hydroxy ketones, RCH(OH)-CO-R, in which R is an alkyl, aryl, or a heterocyclic group, have the class name ‘acyloins’ and are named by substitutive nomenclature as substituted ketones, in accordance with the seniority order: ketones > hydroxy compounds (see P-41). Names ending in ‘oin’ are not recommended.
Examples:
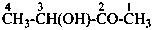
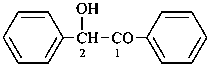
2-hydroxy-1,2-diphenylethan-1-one (PIN)
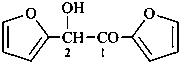
1,2-di(furan-2-yl)-2-hydroxyethan-1-one (PIN)
1,2-di(2-furyl)-2-hydroxyethan-1-one
P-65.0 IntroductionP-65.0 INTRODUCTION
P-65.1 Carboxylic acids and functional replacement analogues
P-65.2 Carbonic, cyanic, and di- and polycarbonic acids
P-65.3 Sulfur, selenium, and tellurium acids with chalcogen atoms directly linked to a parent hydride
P-65.4 Acyl groups as substituent groups
P-65.5 Acyl halides and pseudohalides
P-65.6 Salts and esters
P-65.7 Anhydrides and their analogues
This Chapter includes acids named substitutively by means of suffixes, that is, carboxylic acids, sulfonic, sulfinic, and analogous selenium and tellurium acids. Their derivatives, such as esters, acyl halides, and anhydrides, are also included. Salts are included in this Section although anions are formally treated in Chapter P-7. Carbon acids not named substitutively, i.e., carbonic acid, cyanic acid, and the di- and polynuclear carbon acids are also included here. Mononuclear and polynuclear inorganic (noncarbon) acids used as parent structures for organic derivatives are discussed in Section P-67.
The hydrogen atom of an acid group is not substitutable for the purposes of substitutive nomenclature; replacement of acid hydrogen atoms by specific atoms or groups is called ‘functionalization’, as other classes are generated, for example esters. Substitution takes place when other hydrogen atoms in the structure are exchanged with other atoms or groups, as illustrated by the name ‘chloroacetic acid’.
P-65.1 CARBOXYLIC ACIDS AND FUNCTIONAL REPLACEMENT ANALOGUES
Carboxylic acids have the structure R-C(=O)-OH, where R can be a hydrogen atom. Nitrogenous analogues are carboxylic acids in which =O has been replaced by =NH, =NNH2, =N-OH, or in which –OH has been replaced by –NH-OH. Chalcogen analogues are carboxylic acids in which one or two oxygen atoms have been replaced by sulfur, selenium, or tellurium atoms.
Names of α-amino acids, as well as carboxylic acids derived from carbohydrates, are not covered extensively in this Chapter. Traditional names are maintained, as recommended in specialized publications (refs. 18, 27), and listed in Chapter P-10 devoted to natural products.
P-65.1.1 Retained namesP-65.1.1 Retained names
P-65.1.2 Systematic names
P-65.1.3 Carboximidic, carbohydrazonic, carbohydroximic, and carbohydroxamic acids
P-65.1.4 Peroxycarboxylic acids
P-65.1.5 Chalcogen analogues of carboxylic acids
P-65.1.6 Amic, anilic, and aldehydic acids
P-65.1.7 Acyl groups derived from carboxylic and related acids
P-65.1.8 Formic acid
Carboxylic acids derived from natural sources were often given trivial names reminiscent of their animal or vegetable origin. In both 1979 and 1993, the list of these trivial names was significantly reduced, systematic names being recommended.
P-65.1.1.1 Retained names as preferred IUPAC names
Only the following five carboxylic acids are retained names and are also preferred IUPAC names. All can be functionalized, but only acetic acid, benzoic acid, and oxamic acid can be substituted according to P-15.1.8.2.1; for substitution rules regarding formic acid, see P-65.1.8. Systematic substitutive names are used to generate acids modified by functional replacement.
HOOC-COOH
oxalic acid (PIN)
ethanedioic acid
CH3-COOH
acetic acid (PIN)
ethanoic acid
C6H5-COOH
benzoic acid (PIN)
benzenecarboxylic acid
H2N-CO-COOH
oxamic acid (PIN)
amino(oxo)acetic acid
P-65.1.1.2.1 The following names are retained, but only for general nomenclature, with substitution according to P-15.1.8.2.1 allowed (see also P-34).
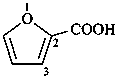
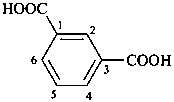
isophthalic acid
benzene-1,3-dicarboxylic acid (PIN)
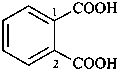
phthalic acid
benzene-1,2-dicarboxylic acid (PIN)
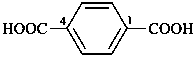
terephthalic acid
benzene-1,4-dicarboxylic acid (PIN)
HOOC-[CH2]4-COOH
adipic acid
hexanedioic acid (PIN)
CH3-CH2-CH2-COOH
butyric acid
butanoic acid (PIN)
C6H5-CH=CH-COOH
cinnamic acid (‘E’ configuration implied)
3-phenylprop-2-enoic acid (PIN;
‘E’ and ‘Z‘ isomers)
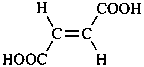
fumaric acid
(2E)-but-2-enedioic acid (PIN)
HOOC-[CH2]3-COOH
glutaric acid
pentanedioic acid (PIN)
HOOC-CH2-COOH
malonic acid
propanedioic acid (PIN)
CH2=C(CH3)-COOH
methacrylic acid
2-methylprop-2-enoic acid (PIN)
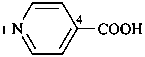
isonicotinic acid
pyridine-4-carboxylic acid (PIN)
maleic acid
(2Z)-but-2-enedioic acid (PIN)
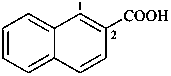
2-naphthoic acid (also 1-isomer)
naphthalene-2-carboxylic acid (PIN)
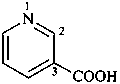
nicotinic acid
pyridine-3-carboxylic acid (PIN)
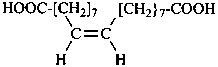
oleic acid
(9Z)-octadec-9-enoic acid (PIN)
CH3-[CH2]14-COOH
palmitic acid
hexadecanoic acid (PIN)
CH3-CH2-COOH
propionic acid
propanoic acid (PIN)
CH3-[CH2]16-COOH
stearic acid
octadecanoic acid (PIN)
HOOC-CH2-CH2-COOH
succinic acid
butanedioic acid (PIN)
CH3-CO-OOH
peracetic acid
ethaneperoxoic acid (PIN)
C6H5-CO-OOH
perbenzoic acid
benzenecarboperoxoic acid (PIN)
H-CO-OOH
performic acid
methaneperoxoic acid (PIN; see P-65.1.4.1)
(HOOC-CH)2N-CH2-CH2-N(CH2-COOH)2
ethylenediaminetetraacetic acid
N,N′-(ethane-1,2-diyl)bis[N-(carboxymethyl)glycine]
2,2′,2′′,2′′′-(ethane-1,2-diyldinitrilo)tetraacetic acid
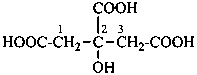
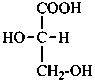
glyceric acid
2,3-dihydroxypropanoic acid (PIN)
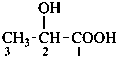
lactic acid
2-hydroxypropanoic acid (PIN)
CH3-CO-COOH
pyruvic acid
2-oxopropanoic acid (PIN)
HOOC-[CH(OH)]2-COOH
tartaric acid
2,3-dihydroxybutanedioic acid (PIN)
(to denote configuration, see P-102.5.6.6.5)
P-65.1.1.2.4 The following trivial names are no longer recommended.
(CH3)2CH-COOH
2-methylpropanoic acid (PIN
(not isobutyric acid)
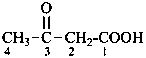
3-oxobutanoic acid (PIN)
(not acetoacetic acid)
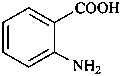
2-aminobenzoic acid (PIN)
[not anthranilic acid (1,2-isomer only)]
(C6H5)2C(OH)-COOH
hydroxydi(phenyl)acetic acid (PIN)
(not benzilic acid)
HO-CH2-COOH
hydroxyacetic acid (PIN)
(not glycolic acid)
OHC-COOH
oxoacetic acid (PIN)
(not glyoxylic acid)
Carboxylic acids are named substitutively using the suffix ‘oic acid’ or ‘carboxylic acid’ and the prefix ‘carboxy’ to describe carboxy groups that cannot be included as the principal characteristic group or to express the carboxy group in the presence of a higher principal characteristic group.
P-65.1.2.1 Carboxylic acid groups, –COOH, that conceptually replace a –CH3 group of methane or terminate an unbranched hydrocarbon chain are named by replacing the final ‘e’ of the name of the corresponding hydrocarbon by the suffix ‘oic acid’. No locants are necessary to denote the positions of the carboxylic acid groups in a hydrocarbon chain; locants are used when hydrocarbon chains are modified by skeletal replacement, as shown in P-15.4.3.2.3. Except for formic acid, acetic acid, oxalic acid (see P-65.1.1.1), and oxamic acid (see P-65.1.1.1), systematically formed names are preferred IUPAC names; the names given in P-65.1.1.2 are retained names for use in general nomenclature.
Examples:
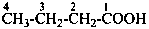
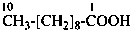
decanoic acid (PIN)
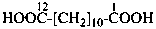
dodecanedioic acid (PIN)
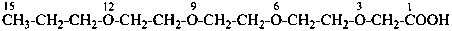
3,6,9,12-tetraoxapentadecan-1-oic acid (PIN)
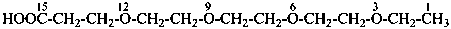
3,6,9,12-tetraoxapentadecan-15-oic acid (PIN)
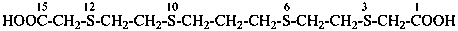
3,6,10,13-tetrathiapentadecane-1,15-dioic acid (PIN)
P-65.1.2.2.1 If an unbranched chain is linked to more than two carboxy groups, all carboxy groups are named from the parent hydride by substitutive use of the suffix ‘carboxylic acid’, preceded by the appropriate numerical prefix ‘tri’, ‘tetra’ etc. and appropriate locants.
Examples:
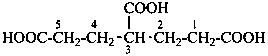
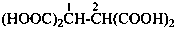
ethane-1,1,2,2-tetracarboxylic acid (PIN)
Examples:
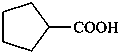
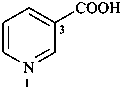
pyridine-3-carboxylic acid (PIN)
nicotinic acid
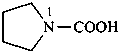
pyrrolidine-1-carboxylic acid (PIN)
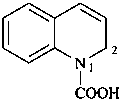
quinoline-1(2H)-carboxylic acid (PIN)
H3Si-O-SiH2-COOH
disiloxanecarboxylic acid (PIN)
H2N-NH-COOH
hydrazinecarboxylic acid (PIN)
carbonohydrazidic acid (see P-65.2.1.4)
(not carbazic acid)
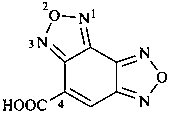
[benzo[1,2-c:3,4-c′]bis([1,2,5]oxadiazole)]-4-carboxylic acid (PIN)
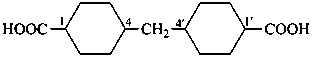
4,4′-methylenedi(cyclohexane-1-carboxylic acid) (PIN)
When another group is present that has priority for citation as suffix, for example, a free valence, or when all carboxylic acid groups cannot be described by a suffix, carboxylic acid groups, –COOH, are indicated by the preferred prefix ‘carboxy’ (also used in general nomenclature). The prefix ‘oxalo’ is recommended as the preferred prefix for –CO-CO-OH, but cannot be used to lengthen a carbon chain. In general nomenclature, the compound prefix ‘carboxycarbonyl’ may be used, but the compound prefix ‘carboxyformyl’ is not recommended.
Examples:
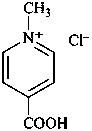
–CH2-CH2-COOH
2-carboxyethyl (preferred prefix)
3-(carboxymethyl)heptanedioic acid (PIN)
1-methyl-3-oxalo-1-azabicyclo[2.2.2]octan-1-ium (PIN)
3-carboxy-3-oxopropyl (preferred prefix)
(not 2-oxaloethyl)
When required, numbering is based on the seniority order given in P-14.4.
Examples:
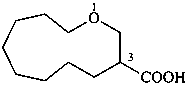
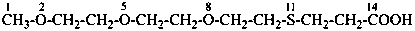
2,5,8-trioxa-11-thiatetradecan-14-oic acid (PIN)
Heteroatoms in chains are now considered to be an integral part of the parent hydride and as such they have seniority over suffixes for numbering (see P-14.4; see also P-15.4).
naphthalene-4a,8a-dicarboxylic acid (PIN; see P-58.2)
4a,8a-dihydronaphthalene-4a,8a-dicarboxylic acid
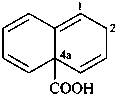
naphthalene-4a(2H)-carboxylic acid (PIN; see P-58.2)
2,4a-dihydronaphthalene-4a-carboxylic acid
Systematic names of substituted carboxylic acids are formed by adding appropriate prefixes, such as ‘oxo’, ‘hydroxy’, ‘amino’, ‘imino’, ‘halo’, ‘nitro’, etc., to the name of the acid. Prefixes are not ranked as functional entities; they are cited in a name in alphabetical order (except for hydro/dehydro) which is also used to assign lowest locants when required.
Examples:
5-aminopentanoic acid (PIN)
3,5-dibromo-4-hydroxybenzoic acid (PIN)
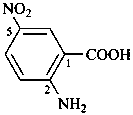
2-amino-5-nitrobenzoic acid (PIN)
(not 5-nitroanthranilic acid;
anthranilic acid is not a retained name)
1-hydroxy-3-oxopropane-1,2,3-tricarboxylic acid (PIN)
[not 3-hydroxy-1-oxopropane-1,2,3-tricarboxylic acid;
lowest locants are attributed to prefixes that are cited first, see P-14.4 (g)]
(HO-CH2-CH2-O)2CH-COOH
bis(2-hydroxyethoxy)acetic acid (PIN)
4-(methylsulfanyl)-2-oxobutanoic acid (PIN)
[not 4-(methylthio)-2-oxobutyric acid]
4-[(hydroxysulfanyl)methyl]benzoic acid (PIN)
[not 4-(sulfenomethyl)benzoic acid]
5,6,7,8-tetrabromo-1,2,3,4-tetrahydroanthracene-9-carboxylic acid (PIN)
(not 1,2,3,4-tetrabromo-5,6,7,8-tetrahydroanthracene-9-carboxylic acid
hydro/dehydro prefixes are given lowest possible locants before other detachable prefixes; see P-14.4)
1-(2-carboxy-2-oxoethyl)-4-hydroxycyclohexa-2,5-diene-1-carboxylic acid (PIN)
1-carboxy-4-hydroxy-α-oxocyclohexa-2,5-dienepropanoic acid
(a conjunctive
name, see P-15.6)
4-hydroxy-6-oxoocta-2,4-dienedioic acid (PIN)
(not 5-hydroxy-3-oxoocta-4,6-dienedioic acid;
unsaturation is senior to detachable prefixes for numbering)
2,2′,2′′,2′′′-(ethane-1,2-diyldinitrilo)tetraacetic acid (see P-15.3.2.1)
N,N′-(ethane-1,2-diyl)bis[N-(carboxymethyl)glycine]
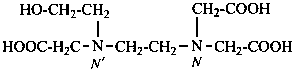
N-(carboxymethyl)-N′-(2-hydroxyethyl)-N,N′-(ethane-1,2-diyl)diglycine
2,2′-({2-[(carboxymethyl)(2-hydroxyethyl)amino]ethyl}azanediyl)diacetic acid
P-65.1.3.1 Carboximidic acids
P-65.1.3.1.1 Substitutive nomenclature, suffix mode
The name of an acid in which the carbonyl oxygen atom of a carboxylic acid group has been replaced by =NH is formed by functional replacement nomenclature and the infix ‘imid(o)’ to modify the ‘ic acid’ or ‘oic acid’ ending of the retained name of an acid; or the ‘oic acid’ or ‘carboxylic acid’ suffix of a systematic name of an acid, to ‘imidic acid’ or ‘carboximidic acid’.
Preferred names of imidic acids are those derived from systematic substitutive preferred IUPAC names of carboxylic acids.
The use of systematic substitutive names for imidic acids is a change for formic acid, acetic acid, benzoic acid, and oxalic acid.
Examples:
CH3-C(=NH)-OH
ethanimidic acid (PIN)
acetimidic acid
C6H5-C(=NH)-OH
benzenecarboximidic acid (PIN)
benzimidic acid
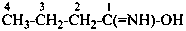
butanimidic acid (PIN)
butyrimidic acid
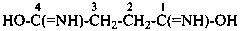
butanediimidic acid (PIN)
succinimidic acid
HO-C(=NH)-C(=NH)-OH
ethanediimidic acid (PIN)
oxalimidic acid
cyclohexanecarboximidic acid (PIN)
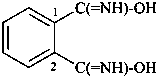
benzene-1,2-dicarboximidic acid (PIN)
phthalimidic acid
When another group is present that has seniority for citation as principal group, the following prefixes are used:
(1) the compound prefix ‘C-hydroxycarbonimidoyl’ used to denote the acyl group –C(=NH)-OH is formed by concatenation based on the simple prefix carbonimidoyl, –C(=NH)–, derived from carbonimidic acid (see P-65.2.1.5);Examples:(2) a combination of the simple prefixes ‘hydroxy’ and ‘imino’ at the end of a carbon chain is used in preferred IUPAC names rather than the compound prefix ‘C-hydroxycarbonimidoyl’.
Note 1: The italicized letter ‘C’ is used to avoid potential confusion with N-hydroxy substitution.
Note 2: The name carbonohydroximoyl, for –C(=NH)-OH, is not used to generate preferred IUPAC names.
(1) 4-(C-hydroxycarbonimidoyl)benzoic acid (PIN)
(2) 4-hydroxy-4-iminobutanoic acid (PIN)
(1) 3-(C-hydroxycarbonimidoyl)propanoic acid
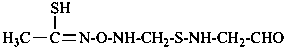
N-{[({[(2-oxoethyl)amino]sulfanyl}methyl)amino]oxy}ethanimidothioic acid (PIN)
(see P-65.1.5.2)
P-65.1.3.2.1 Substitutive nomenclature, suffix mode
The name of an acid in which the carbonyl oxygen atom of a carboxylic acid group has been replaced by =NNH2 is formed by functional replacement nomenclature. The infix ‘hydrazon(o)’ is used to modify the ‘ic acid’ or ‘oic acid’ ending of the retained name of an acid; or the ‘oic acid’ or ‘carboxylic acid’ suffix of a systematic name of an acid is changed to ‘hydrazonic acid’ or ‘carbohydrazonic acid’.
Preferred IUPAC names for hydrazonic acids are those derived from systematic preferred IUPAC names of carboxylic acids.
The use of systematic substitutive names for hydrazonic acids is a change for formic acid, acetic acid, benzoic acid, and oxalic acid.
Examples:
CH3-C(=N-NH2)-OH
ethanehydrazonic acid (PIN)
acetohydrazonic acid
C6H5-C(=N-NH2)-OH
benzenecarbohydrazonic acid (PIN)
benzohydrazonic acid
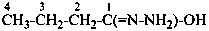
butanehydrazonic acid (PIN)
butyrohydrazonic acid
butanedihydrazonic acid (PIN)
succinohydrazonic acid
HO-C(=N-NH2)-C(=N-NH2)-OH
ethanedihydrazonic acid (PIN)
oxalohydrazonic acid
cyclohexanecarbohydrazonic acid (PIN)
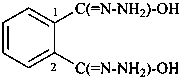
benzene-1,2-dicarbohydrazonic acid (PIN)
phthalohydrazonic acid
When another group is present that has seniority for citation as principal group, the following prefixes are used:
(1) the compound prefix ‘C-hydroxycarbonohydrazonoyl’ used to denote the acyl group –C(=N-NH2)-OH is formed by concatenation based on the simple prefix name carbonohydrazonoyl, –C(=NNH2)–, derived from carbonohydrazonic acid (see P-65.2.1.5)]. The substitutive name hydrazinylidene(hydroxy)methyl may be used in general nomenclature;Examples:(2) the combination of the simple prefixes ‘hydroxy’ and ‘hydrazinylidene’ at the end of a carbon chain is used in preferred IUPAC names rather than the compound prefixes ‘C-hydroxycarbonohydrazonoyl’ or ‘hydrazinylidene(hydroxy)methyl’.
Note: The italicized letter ‘C’ is used to avoid potential confusion with N-hydroxy substitution.
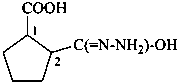
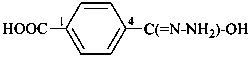
(1) 4-(C-hydroxycarbonohydrazonoyl)benzoic acid (PIN)
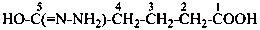
(2) 5-hydrazinylidene-5-hydroxypentanoic acid (PIN)
(1) 4-(C-hydroxycarbonohydrazonoyl)butanoic acid
P-65.1.3.3.1 Substitutive nomenclature, suffix mode
Acids in which the carbonyl oxygen atom of a carboxylic acid group has been replaced by =N-OH are named as N-hydroxy derivatives of imidic acids named as described in P-65.1.3.1. This method is used to generate preferred IUPAC names.
Note: The following former methodology is no longer used for preferred IUPAC names:
The name formed by modifying the ‘-oic acid’ or ‘-carboxylic acid’ suffix of a systematically named acid, or the ‘-ic acid’ ending of the retained name of an acid to ‘-hydroximic acid’ or ‘-carbohydroximic acid’. The letter ‘o’ is added for euphony between ‘h’ and a preceding consonant.This former method may be still used in general nomenclature.
Examples:
C6H5-C(=N-OH)-OH
N-hydroxybenzenecarboximidic acid (PIN)
benzohydroximic acid
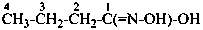
N-hydroxybutanimidic acid (PIN)
butyrohydroximic acid
butanohydroximic acid
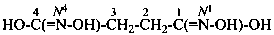
N1,N4-dihydroxybutanediimidic acid
succinohydroximic acid
butanedihydroximic acid
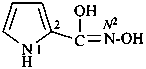
N2-hydroxy-1H-pyrrole-2-carboximidic acid (PIN)
pyrrole-2-carbohydroximic acid
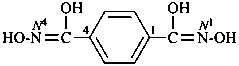
N1,N′4-dihydroxybenzene-1,4-dicarboximidic acid (PIN)
terephthalohydroximic acid
When another group is present that has seniority for citation as principal group, the following prefixes are used:
(1) ‘C,N-dihydroxycarbonimidoyl’ to denote the group –C(=N-OH)-OH;Examples:(2) the combination of the prefixes ‘hydroxy’ and ‘hydroxyimino’ at the end of a carbon chain is used in preferred IUPAC names rather than the prefix ‘dihydroxycarbonimidoyl’.
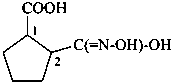
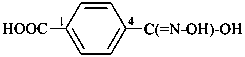
(1) 4-(C,N-dihydroxycarbonimidoyl)benzoic acid (PIN)
(2) 5-hydroxy-5-(hydroxyimino)pentanoic acid (PIN)
(1) 4-(C,N-dihydroxycarbonimidoyl)butanoic acid
Examples:
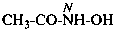
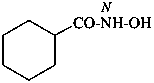
N-hydroxycyclohexanecarboxamide (PIN)
cyclohexanecarbohydroxamic acid
The general methodology for modifying acids expressed by suffixes by functional replacement nomenclature is to use modified suffixes in the same way as for unmodified acids. A major change and simplification is recommended, i.e., suffixes are always modified by infixes.
P-65.1.4.1 Peroxycarboxylic acids are named systematically using the following suffixes:
–(C)O-OOH peroxoic acidRetained names of carboxylic acids are modified by the prefix ‘peroxy’. Preferred IUPAC names are formed by functional replacement of systematic carboxylic acid names.–CO-OOH carboperoxoic acid
The use of systematic substitutive names for peroxycarboxylic acids is a change for formic acid, acetic acid, benzoic acid, oxalic acid, and oxamic acid.
Examples:
CH3-CO-OOH
ethaneperoxoic acid (PIN)
peroxyacetic acid
peracetic acid
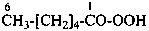
hexaneperoxoic acid (PIN)
C6H5-CO-OOH
benzenecarboperoxoic acid (PIN)
peroxybenzoic acid
perbenzoic acid
H2N-CO-CO-OOH
amino(oxo)ethaneperoxoic acid (PIN)
peroxyoxamic acid
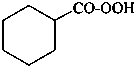
cyclohexanecarboperoxoic acid (PIN)
HOO-CO-CO-OOH
ethanediperoxoic acid (PIN)
diperoxyoxalic acid
(1) the simple functional replacement prefix ‘carbonoperoxoyl-’ or the compound prefix ‘hydroperoxycarbonyl-’, formed by concatenation based on the simple acyl group ‘carbonyl’, for >C=O (see P-65.2.1.5) is used to denote the acyl group –C(O)-OOH as a substituent; the prefix ‘carbonoperoxoyl’ is used in preferred IUPAC names, except as noted below in (2);Examples:(2) the combination of the simple prefixes ‘hydroperoxy and oxo’ at the end of a carbon chain is used in preferred IUPAC names rather than the prefix ‘hydroperoxycarbonyl-’ or the prefix ‘carbonoperoxoyl-’.
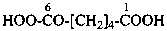
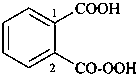
(1) 2-carbonoperoxoylbenzoic acid (PIN)
(2) 2-(hydroperoxycarbonyl)benzoic acid
monoperoxyphthalic acid (see P-65.1.4.1)
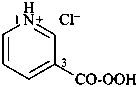
(1) 3-carbonoperoxoylpyridin-1-ium chloride (PIN)
3-(hydroperoxycarbonyl)pyridin-1-ium chloride
P-65.1.5.1 Functional replacement in systematic names of carboxylic acids
Replacement of oxygen atom(s) of a carboxylic acid group by another chalcogen is indicated by the affixes ‘thio’, ‘seleno’, and ‘telluro’. These names do not differentiate between tautomeric forms of mixed chalcogen acids; such nonspecificity may be shown in a structure such as:
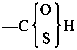 | or –C{O/S}H |
When the position of chalcogen atoms is undetermined, the prefix for the unmodified acid, i.e. ‘carboxy’ for –COOH, is used and modified by functional replacement using prefixes, as in ‘thiocarboxy’ for –C{O/S}H, and is enclosed in parentheses to avoid the possibility of ambiguity. The order of seniority of these suffixes is fully described in Section P-43.
When the position of chalcogen atoms is known, combinations of prefixes such as ‘hydroxy- and sulfanylidene-’ or ‘sulfanyl- and oxo-’ are used in acyclic compounds. Compound prefixes such as ‘(hydroxycarbonothioyl)-’ and ‘(sulfanylcarbonyl)-’ are used when required as a substituent (see P-65.2.1.6). The compound prefixes are formed by concatenation using simple acyl prefixes derived from carbonic acids (see P-65.2.1.5)
The seniority order between acids and acids modified by functional replacement is discussed in P-43 and expressed in Table 4.3. In presence of unmodified acids cited as suffix, modified acids are cited as prefixes.
Examples:
CH3-CH2-CH2-CH2-CH2-C{S/Se}H
hexaneselenothioic acid (PIN)
CH3-CH2-CH2-CH2-CH2-CSe-SH
hexaneselenothioic S-acid (PIN)
H{S/O}C-CH2-CH2-CH2-CH2-C{O/S}H
hexanebis(thioic acid) (PIN)
HS-SC-CH2-CH2-CH2-CH2-CS-SH
hexanebis(dithioic acid) (PIN)
CH3-CH2-CH2-CH2-CH2-C{O/Se}H
hexaneselenoic acid (PIN)
H{S/O}C-CH2-CH2-C{O/S}H
butanebis(thioic acid) (PIN)
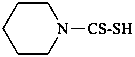
piperidine-1-carbodithioic acid (PIN)
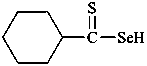
cyclohexanecarboselenothioic Se-acid (PIN)
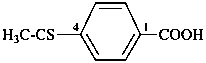
4-(ethanethioyl)benzoic acid (PIN)
4-(thioacetyl)benzoic acid
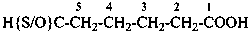
5-(thiocarboxy)pentanoic acid (PIN)
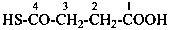
4-oxo-4-sulfanylbutanoic acid (PIN)
3-(sulfanylcarbonyl)propanoic acid
HO-CS-CH2-CH2-COOH
4-hydroxy-4-sulfanylidenebutanoic acid (PIN)
HS-CO-COOH
oxo(sulfanyl)acetic acid (PIN)
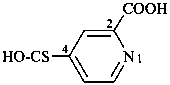
4-(hydroxycarbonothioyl)pyridine-2-carboxylic acid (PIN)
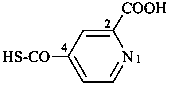
4-(sulfanylcarbonyl)pyridine-2-carboxylic acid (PIN)
CH3-CH2-C(=NH)-SH
propanimidothioic acid (PIN)
CH3-CH2-CH2-C(=NNH2)-SeH
butanehydrazonoselenoic acid (PIN)
N-sulfanylcyclopentanecarboximidic acid (PIN)
N-hydroxycyclohexanecarboximidoselenoic acid (PIN)
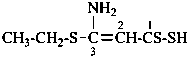
3-amino-3-(ethylsulfanyl)prop-2-ene(dithioic acid) (PIN)
Preferred names of chalcogen analogues of monocarboxylic acids are formed using the suffixes ‘thioic acid’, ‘selenoic acid’, ‘telluroic acid’ or ‘carbothioic acid’, ‘carboselenoic acid’, ‘carbotelluroic acid’ and names of appropriate parent hydrides, even in the case of formic acid, acetic acid, and benzoic acid.
The use of systematic substitutive names for chalcogen analogues of monocarboxylic acids is a change for formic acid, acetic acid, benzoic acid, oxalic acid, and oxamic acid.
Chalcogen analogues of monocarboxylic acids with retained names may also be named by placing the prefix ‘thio’, ‘seleno’, or ‘telluro’ in front of the name of the acid.
Chalcogen analogues of dicarboxylic acids are named systematically; retained names are not used for naming chalcogen analogues of dicarboxylic acids.
The symbols O, S, Se, and Te are used to specify the structure of the acid, as indicated in P-65.1.5.1.
Examples:
C6H5-C{O/Se}H
benzenecarboselenoic acid (PIN)
selenobenzoic acid
HCO-SH
methanethioic S-acid (PIN)
thioformic S-acid
H2N-CO-CO-C{O/S}H
3-amino-2,3-dioxopropanethioic acid (PIN)
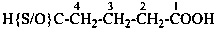
4-(thiocarboxy)butanoic acid (PIN)
(not thioglutaric acid)
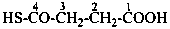
4-oxo-4-sulfanylbutanoic acid (PIN)
(not thiosuccinic acid)
H{S/O}C-COOH
(thiocarboxy)formic acid
(see P-65.1.8.2)
HO-CS-COOH
hydroxy(sulfanylidene)acetic acid (PIN)
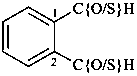
benzene-1,2-dicarbothioic acid (PIN)
(not 1,2-dithiophthalic acid)
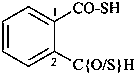
2-(thiocarboxy)benzene-1-carbothioic S-acid (PIN)
(not 1,2-dithiophthalic S-acid)
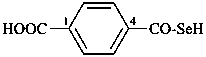
4-(selanylcarbonyl)benzoic acid (PIN)
(not selenoterephthalic Se-acid)
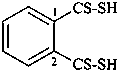
benzene-1,2-dicarbodithioic acid (PIN)
(not tetrathiophthalic acid)
HS-CS-CS-SH
ethanebis(dithioic acid) (PIN)
(not tetrathiooxalic acid)
Peroxy acid suffixes can be modified by S, Se, and Te using functional replacement nomenclature. Italic prefixes in front of the term ‘acid’ are used for specificity, where necessary (see Table 4.3 for more suffixes modified by functional replacement and their seniority order). Preferred names are all formed by using appropriate suffixes and parent hydrides, even in the case of derivatives of formic acid, acetic acid, and benzoic acid.
The use of systematic substitutive names for chalcogen analogues of peroxycarboxylic acids is a change for formic acid, acetic acid, benzoic acid, and oxalic acid.
Examples:
–(C)O-OSH (thioperoxoic) OS-acid (preferred suffix)The recommended suffixes, and their seniority order, are fully discussed in Section P-43.–(C)Se-SSH (dithioperoxo)selenoic acid (preferred suffix)
–CO-SOH carbo(thioperoxoic) SO-acid (preferred suffix)
–CS-OOH carboperoxothioic acid (preferred suffix)
–COS2H dithiocarboperoxoic acid (preferred suffix; location of sulfur atom unknown)
Examples:
C6H5-CO-SOH
benzenecarbo(thioperoxoic) SO-acid (PIN)
(not peroxothiobenzoic SO-acid)
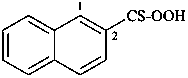
naphthalene-2-carboperoxothioic acid (PIN)
(not peroxythio-2-naphthoic acid)
Prefixes derived by functional replacement nomenclature have only limited use because there is no accepted method to unambiguously describe precise structures of thioperoxy groups.
Examples:
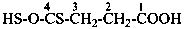
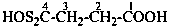
3-(dithiocarbonoperoxoyl)propanoic acid (PIN)
(location of sulfur atoms unknown)
HOS2C-COOH
(dithiocarbonoperoxoyl)formic acid (PIN)
(location of sulfur atoms unkown)
HOS-CO-COOH
(hydroxysulfanyl)oxoacetic acid (PIN)
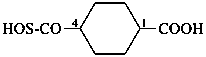
4-[(hydroxysulfanyl)carbonyl]cyclohexane-1-carboxylic acid (PIN)
4-[(OS-thiohydroperoxy)carbonyl]cyclohexanecarboxylic acid
Amic acids are compounds containing both a carboxy, –COOH, and a carboxamide, –CONH2, group; similarly, anilic and aldehydic acids include both a carboxy group and a carboxanilide, –CO-NH-C6H5, or formyl, –CHO, group, respectively. The endings ‘amic acid’, ‘anilic acid’, and ‘aldehydic acid’ can only be used in general nomenclature to name modified dicarboxylic acids having retained names. Preferred IUPAC names are all formed systematically using preferred names of acids and appropriate prefixes.
P-65.1.6.1 Amic acids
When a dicarboxylic acid has a retained name (see P-65.1.1) and when one of its carboxy groups is replaced by a carboxamide group, –CO-NH2, the resulting structure is called an amic acid and, in general nomenclature may be named by replacing the ending ‘ic acid’ of the name of the dicarboxylic acid by the ending ‘amic acid’. The case of oxalic acid is special; substitution is not possible for the acid, but substitution is allowed for the derived amic acid, ‘oxamic acid’ but no ‘N’ locamnts are necessary. The name ‘oxamic acid’ (a contraction of ‘oxalamic acid’) is retained for H2N-CO-COOH and is the preferred IUPAC name.
The prefix ‘carbamoyl’ is preferred to ‘aminocarbonyl’ for naming amic acids systematically. The combination of the prefixes ‘amino’ and ‘oxo’ is used for describing the –CO-NH2 at the end of an acyclic chain in preferred IUPAC names
Examples:
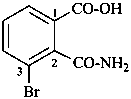
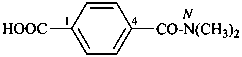
4-(dimethylcarbamoyl)benzoic acid (PIN)
4-[(dimethylamino)carbonyl]benzoic acid
N,N-dimethylterephthalamic acid
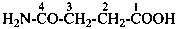
4-amino-4-oxobutanoic acid (PIN)
3-carbamoylpropanoic acid
3-(aminocarbonyl)propanoic acid
succinamic acid
H2N-CO-COOH
oxamic acid (PIN; a retained name)
(not oxalamic acid)
N-Phenyl derivatives of amic acids are called ‘anilic acids’ and in general nomenclature are named by changing an ‘amic acid’ ending to ‘anilic acid’. Substitution on the nitrogen atom is indicated by the locant N, even if no substitution is allowed on the parent acid. Anilic acids may also be named as N-substituted amic acids. The locants for substituents on the N-phenyl ring are primed numbers.
The combination of the prefixes ‘anilino’ and ‘oxo’ is used for describing –CO-NH-C6H5 at the end of an acyclic chain resulting in preferred IUPAC names
Examples:
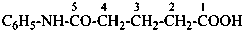
C6H5-NH-CO-COOH
anilino(oxo)acetic acid (PIN)
oxalanilic acid
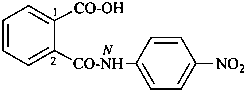
2-[(4-nitrophenyl)carbamoyl]benzoic acid (PIN)
N-(4-nitrophenyl)phthalamic acid
4′-nitrophthalanilic acid
When a dicarboxylic acid has a retained name (see P-65.1.1) and when one of its carboxy groups is replaced by a formyl group, –CHO (see P-65.1.7.2.1), the resulting structure is called an aldehydic acid and, in general nomenclature may be named by replacing the ending ‘ic acid’ of the name of the dicarboxylic acid by the ending ‘aldehydic acid’. Preferred IUPAC names for aldehydic acids derived from all dicarboxylic acids are constructed systematically. The prefix ‘formyl’ is used in preferred IUPAC names, except for a –CHO group at the end of an acyclic chain, which is designated by the prefix ‘oxo’.
Examples:
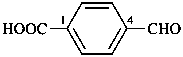
4-oxobutanoic acid (PIN)
3-formylpropanoic acid
succinaldehydic acid
OCH-CO-OH
oxoacetic acid (PIN)
(not glyoxylic acid)
P-65.1.7.1 Definitions and name formationP-65.1.7.1 Definitions and name formation
P-65.1.7.2 Acyl groups derived from carboxylic acids having retained names that are preferred IUPAC names (see P-65.1.1.1), i.e., carboacyl groups
P-65.1.7.3 Acyl groups derived from carboxylic acids with names retained only for general nomenclature (see P-65.1.1.2)
P-65.1.7.4 Acyl groups derived from systematically named carboxylic acids
P-65.1.7.5 Mixed acyl groups
Carboacyl groups are R-CO–, –OC-R-CO–, or –OC-R-[R′-CO-]x-R′′-CO– groups and their functional replacement analogues, where R, R′, and R′′ are chains, rings, or ring systems, derived from carboxylic acids by the removal of the hydroxy group from each carboxylic acid group that is expressed by the suffix, and x = 1, 2, 3, etc.
Systematic names for carboacyl groups and their functional replacement analogues are given in the following subsections. Compound substitutive names for acyclic acyl groups, such as ‘1-oxopropyl’ and ‘1-iminoethyl’ for CH3-CH2-CO– and CH3-C(=NH)–, respectively, are included for use in general nomenclature.
P-65.1.7.2 Acyl groups derived from carboxylic acids having retained names that are preferred IUPAC names (see P-65.1.1.1), i.e., carboacyl groups.
The name of a monovalent or divalent carboacyl groups derived by removal of the –OH group from each carboxy group of a carboxylic acid or functional replacement analogue denoted by an ‘oic acid’ or ‘ic acid’ suffix or having a trivial name is derived from the name of the corresponding acid by changing the ‘oic acid’ or ‘ic acid’ ending to ‘oyl’ or ‘yl’. The general rule that the ending of all acyl group prefixes be ‘oyl’, proposed years ago, has not been regularly followed. This rule is fully implemented in these recommendations, but some traditional exceptions are maintained.
Carboacyl groups derived from acids named by means of the suffix ‘carboxylic acid’ are named by changing the suffix ‘carboxylic acid’ to ‘carbonyl’. Acyl groups derived from functional replacement analogues are named by changing the suffixes ‘carbothioic acid’ to ‘carbothioyl’ (and likewise for the selenium and tellurium analogues); ‘carboximidic acid’ to ‘carboximidoyl’; ‘carbohydrazonic acid’ to ‘carbohydrazonoyl’; and ‘carbohydroximic acid’ to ‘carbohydroximoyl’.
P-65.1.7.2.1 Acyl groups from the carboxylic acids that have retained names used as preferred IUPAC names (see P-65.1.1.1)
Examples:
HCO–
formyl (preferred prefix)
methanoyl
oxomethyl
C6H5-CO–
benzoyl (preferred prefix)
benzenecarbonyl
oxo(phenyl)methyl
–CO-CO–
oxalyl (preferred prefix)
ethanedioyl
dioxoethanediyl
HO-CO-CO–
oxalo (preferred prefix)
carboxycarbonyl
[not carboxyformyl;
not hydroxy(oxo)acetyl]
The use of systematically derived acyl groups from imidic, hydrazonic, hydroximic, and hydroxamic acids is a changes for formic, acetic, benzoic, and oxalic acids.
Examples:
HC(=NH)–
methanimidoyl (preferred prefix)
formimidoyl
iminomethyl
C6H5-C(=NH)–
benzenecarboximidoyl (preferred prefix)
benzimidoyl
imino(phenyl)methyl
–C(=NH)-C(=NH)–
ethanediimidoyl (preferred prefix)
oxalimidoyl
diiminoethanediyl
HC(=NNH2)–
methanehydrazonoyl (preferred prefix)
formohydrazonoyl
hydrazinylidenemethyl
CH3-C(=NNH2)–
ethanehydrazonoyl (preferred prefix)
acetohydrazonoyl
1-hydrazinylideneethyl
C6H5-C(=N-OH)–
N-hydroxybenzenecarboximidoyl (preferred prefix)
N-hydroxybenzimidoyl
benzenecarbohydroximoyl
The use of systematically derived acyl groups from chalcogen analogues of carboxylic acids is a change for formic, acetic, benzoic, and oxalic acids.
Examples:
HCS–
methanethioyl (preferred prefix)
thioformyl
sulfanylidenemethyl
C6H5-CS–
benzenecarbothioyl (preferred prefix)
thiobenzoyl
–CS-CS–
ethanebis(thioyl) (preferred prefix)
dithiooxalyl
bis(sulfanylidene)ethanediyl
Examples:
Cl-CO-CO–
chloro(oxo)acetyl (preferred prefix)
chlorooxalyl
HO-CO-CS–
carboxymethanethioyl (preferred prefix)
HO-CS-CO–
hydroxy(sulfanylidene)acetyl (preferred prefix)
(not 2-thiooxalo;
not 2-hydroxy-2-thiooxalyl)
HO-CS-CS–
hydroxy(sulfanylidene)ethanethioyl (preferred prefix)
hydroxybis(sulfanylidene)ethyl
(not 1,2-dithiooxalyl)
HS-CS-CS–
sulfanyl(sulfanylidene)ethanethioyl (preferred prefix)
trithiooxalo
HO-CO-CO-O–
oxalooxy (preferred prefix)
(carboxycarbonyl)oxy
HO-CO-CO-NH–
oxaloamino (preferred prefix)
(carboxycarbonyl)amino
HO-CO-CO-S–
oxalosulfanyl (preferred prefix)
(carboxycarbonyl)sulfanyl
HO-CO-CS-S–
(carboxymethanethioyl)sulfanyl (preferred prefix)
P-65.1.7.3.1 Traditional names are maintained for acyl groups derived from acids having retained names for use only in general nomenclature (see P-65.1.1.2); substitution on acyl groups is identical to that of acids. The rule of having acyl groups ending in ‘oyl’ is applied, with certain exceptions that end in ‘yl’. The following exceptions below are limiting. Preferred IUPAC names are systematic substitutive names.
Examples:
CH3-CH2-CO–
propionyl
propanoyl (preferred prefix)
1-oxopropyl
–OC-CH2-CO–
malonyl
propanedioyl (preferred prefix)
1,3-dioxopropane-1,3-diyl
–CO-CH2-CH2-CO–
succinyl
butanedioyl (preferred prefix)
1,4-dioxobutane-1,4-diyl
–OC-[CH2]3-CO–
glutaryl
pentanedioyl (preferred prefix)
1,5-dioxopentane-1,5-diyl
CH2=CH-CO–
acryloyl
prop-2-enoyl (preferred prefix)
1-oxoprop-2-en-1-yl
CH2=C(CH3)-CO–
methacryloyl
2-methylprop-2-enoyl (preferred prefix)
2-methyl-1-oxoprop-2-en-1-yl
phthaloyl
benzene-1,2-dicarbonyl (preferred prefix)
1,2-phenylenebis(oxomethylene)
Examples:
CH2=CH-C(=NNH2)–
acrylohydrazonoyl
prop-2-enehydrazonoyl (preferred prefix)
1-hydrazinylideneprop-2-en-1-yl
–(HN=)C-CH2-CH2-C(=NH)–
succinimidoyl
butanediimidoyl (preferred prefix)
1,4-diiminobutane-1,4-diyl
terephthalimidoyl
benzene-1,4-dicarboximidoyl (preferred prefix)
1,4-phenylenebis(iminomethylene)
Names of acyl groups derived from monocarboxylic acids are modified by prefixes expressing functional replacement by =S, =Se, and =Te. Acyl group prefixes corresponding to dicarboxylic acids are formed systematically, in accordance with Rule P- 65.1.7.4.
Examples:
CH2=CH-CSe–
selenoacryloyl
prop-2-eneselenoyl (preferred prefix)
1-selanylideneprop-2-en-1-yl
HS-CS-CS–
sulfanyl(sulfanylidene)ethanethioyl (preferred prefix)
2-sulfanyl-1,2-bis(sulfanylidene)ethyl
trithiooxalo
P-65.1.7.4.1 The name of a monovalent or divalent acyl group formed by removal of the -OH group from each carboxy group of a carboxylic acid denoted by an ‘oic acid’ suffix is derived from the name of the corresponding acid by changing the ending ‘oic acid’ to ‘oyl’. Names of acyl groups derived from carboxylic acids modified by functional replacement are all denoted by the ending ‘oyl’.
Examples:
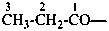
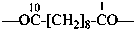
decanedioyl (preferred prefix)
1,10-dioxodecane-1,10-diyl
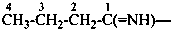
butanimidoyl (preferred prefix)
butyrimidoyl
1-iminobutyl
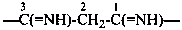
propanediimidoyl (preferred prefix)
malonimidoyl
1,3-diiminopropane-1,3-diyl
CH3-CH2-CS–
propanethioyl (preferred prefix)
thiopropionyl
1-sulfanylidenepropyl
1-thioxpropyl
–CS-CH2-CH2-CS–
butanebis(thioyl) (preferred prefix)
1,4-bis(sulfanylidene)butane-1,4-diyl
1,4-dithioxobutane-1,4-diyl
(not dithiosuccinyl)
Examples:
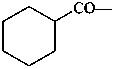
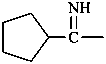
cyclopentanecarboximidoyl (preferred prefix)
cyclopentylcarbonimidoyl
cyclopentyl(imino)methyl
cyclohexane-1,2-dicarbothioyl (preferred prefix)
1-methylcyclopentane-1-carbohydrazonoyl (preferred prefix)
hydrazinylidene(1-methylcyclopentyl)methyl
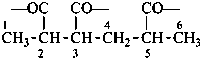
hexane-2,3,5-tricarbonyl (preferred prefix)
hexane-2,3,5-triyltris(oxomethylene)
hexane-2,3,5-tris(carbonyl)
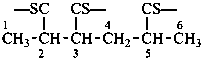
hexane-2,3,5-tricarbothioyl (preferred prefix)
hexane-2,3,5-triyltris(sulfanylidenemethylene)
hexane-2,3,5-triyltris(thioxomethylene)
Examples:
benzene-1,2-dicarbothioyl (preferred prefix)
(not dithiophthaloyl)
1,2-phenylenebis(sulfanylidenemethylene)
1,2-phenylenebis(thioxomethylene)
Mixed acyl groups of the type –(C=X)-[CH2]x-(C=Y)– are named by substitution of alkanediyl substituent groups.
Examples:
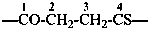
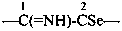
1-imino-2-selanylideneethane-1,2-diyl (preferred prefix)
For the purpose of organic nomenclature, formic acid is considered to be a monocarboxylic acid (see P-65.1). It is a retained name, treated like acetic acid, can be functionalized leading to salts, esters, and anhydrides, and forms an acyl group that is used as a substituent group. Functional replacement analogues are named systematically, for example, methanethioic acid and methanimidic acid. The hydrogen atom attached to carbon is substitutable under specific conditions that are described in P-65.1.8.1, P- 65.1.8.2, and P-65.1.8.3.
P-65.1.8.1 Substitution of the hydrogen atom of formic acid by the following atoms or groups is not recommended:
Note: Substitution of the hydrogen atom of formic acid by –NH-NH2 leads to a structure named by the suffix carboxylic acid attached to the parent hydride hydrazine (see P-68.3.1.2). A carboxylic acid named by means of a suffix is senior to a derivative of carbonic acid formed by functional replacement (see P-41).
Examples:
Cl-COOH
carbonochloridic acid (PIN)
(not chloroformic acid)
HS-COOH
carbonothioic S-acid (PIN)
(not sulfanylformic acid)
Examples:
H{S/O}C-COOH
(thiocarboxy)formic acid (PIN, see P-65.1.5.2)
Examples:
Br-CS–
carbonobromidothioyl (preferred prefix)
[not bromo(thioformyl)]
HCO-O–
formyloxy (preferred prefix)
HCO-S–
formylsulfanyl (preferred prefix)
Carbonic acid, cyanic acid, and di- and polycarbonic acids are a group of functional parent compounds different from carboxylic acids; these acids have no hydrogen atom(s) to be used in substitutive nomenclature.
The following acids, classified as mononuclear carbon acids, have retained names that are preferred IUPAC names:
carbonic acid (PIN) HO-CO-OHThe following di- or polynuclear carbon acids have retained names that are preferred IUPAC names:cyanic acid (PIN) HO-CN
dicarbonic acid (PIN) HO-CO-O-CO-OHExample:tricarbonic acid (PIN) HO-CO-O-CO-O-CO-OH
tetracarbonic acid (PIN) HO-CO-O-CO-O-CO-O-CO-OH
polycarbonic acids HO-[CO-O]n-H n = 5, 6 and higher homologues are named by skeletal replacement (‘a’) nomenclature
P-65.2.1 Carbonic acidP-65.2.1 Carbonic acid
P-65.2.2 Cyanic acid
P-65.2.3 Di-, tri-, tetra-, and polycarbonic acids
The nomenclature of chalcogen analogues and derivatives of carbonic acid is based on functional replacement of one oxygen in –OH groups or of the doubly bonded oxygen atom, =O, and is indicated by infixes. Substitution of formic acid is not recommended for generation of these names.
P-65.2.1.1 The contracted name ‘carbamic acid’ (from carbonamidic acid), for H2N-CO-OH, and ‘carbamimidic acid’ (from carbonamidimidic acid), for H2N-C(=NH)-OH, are retained and are the preferred IUPAC names.
Examples:
N-ethyl-N-methylcarbamimidic acid (PIN)
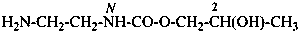
2-hydroxypropyl (2-aminoethyl)carbamate (PIN)
Contrary to Rules P-65.1.3 through P-65.1.5, functional replacement nomenclature is applied to the retained name ‘carbamic acid’ and not to the systematic name ‘carbonamidic acid’.
Examples:
H2N-CO-SeH
carbamoselenoic Se-acid (PIN)
HO-CO-SH
carbonothioic S-acid (PIN)
(not sulfanylformic acid)
HSe-CO-SeH
carbonodiselenoic Se,Se-acid (PIN)
HS-CS-SH
carbonotrithioic acid (PIN)
H2N-CO-OOH
carbamoperoxoic acid (PIN)
HO-CO-OOH
carbonoperoxoic acid (PIN)
HOO-CO-OOH
carbonodiperoxoic acid (PIN)
HO-CO-OSH
carbono(thioperoxoic) OS-acid (PIN)
HOS-CO-OSH
carbonobis(thioperoxoic) OS,SO-acid (PIN)
Italic letter locants N, N′ etc. are used to designate substitution on nitrogen atoms.
Examples:
H2N-C(=NH)-OH
carbamimidic acid (PIN; retained name)
HO-C(=N-NH2)-OH
carbonohydrazonic acid (PIN)
HS-C(=NH)-OH
carbonimidothioic acid (PIN)
H2N-C(=NH)-SH
carbamimidothioic acid (PIN)
HSe-C(=N-NH2)-SeH
carbonohydrazonodiselenoic acid (PIN)
H2N-C(=NH)-OSH
carbamimido(thioperoxoic) OS-acid (PIN)
Italic letter locants N, N′, etc. are used to designate substitution on nitrogen atoms.
Replacement by the –NHNH2 group results in hydrazinecarboxylic acid and related derivatives (see P-68.3.1.2).
Examples:
H2N-C(=NH)-OH
carbamimidic acid (PIN; retained name)
(not carbonamidimidic acid)
H2N-CO-SH
carbamothioic S-acid (PIN)
H2N-C(=NH)-SeH
carbamimidoselenoic acid (PIN)
Cl-CO-OH
carbonochloridic acid (PIN)
NC-CO-OH
carbonocyanidic acid (PIN)
N3-CO-OH
carbonazidic acid (PIN)
SCN-CO-OH
carbonisothiocyanatidic acid (PIN)
H2N-NH-CO-OH
hydrazinecarboxylic acid (PIN)
carbonohydrazidic acid
(carboxylic acids expressed by suffixes are preferred to carbonic acid analogues;
see P-41 and P-68.3.1.2.1)
Acyl groups derived from carbonic and related acids, including functional replacement analogues, by the removal of one or two hydroxy groups from the acid are named in accordance with the methodology described in P-65.1.7.2. Names are formed in two ways:
(1) Names may be formed by changing the -ic or -oic acid ending of the name of the acid to -yl or -oyl, respectively. Names of acyl groups ending in -yl are exceptions to the general rule (see P-65.1.7.2). This method is the traditional method that consists in removing the two hydroxy groups from carbonic acid or its analogues; it is now recommended to be used also when only one hydroxy group is present in an acid. It is also recommended that divalent acyl groups, such as ‘carbonyl’ represent only the ‘diyl’ type of substituent prefix in which the two free valences are divergent (symbols CO< or –CO–). Substituent prefixes in which both free valences are attached to the same atom are named by substitutive nomenclature, for example, =CO is named oxomethylidene (see P-65.2.1.8);Names formed by method (1) are preferred IUPAC names; they are preferred to other names for acyl groups, including the use of prefixes rather than infixes or names formed by full or partial concatenation.(2) Names may be formed by a concatenation operation, i.e., by adding appropriate monovalent substituent groups to divalent acyl groups such as ‘carbonyl’, ‘carbonothioyl’, and ‘carbonimidoyl’ formed by method (1).
Examples:
| HO-CO-OH carbonic acid (PIN) | –CO– carbonyl (preferred prefix) |
| HO-CS-OH carbonothioic O,O-acid (PIN) | –CS– carbonothioyl (preferred prefix) thiocarbonyl |
| HO-C(=NH)-OH carbonimidic acid (PIN) | –C(=NH)– carbonimidoyl (preferred prefix) |
| HO-C(=NNH2)-OH carbonohydrazonic acid (PIN) | –C(=N-NH2)– carbonohydrazonoyl (preferred prefix) |
| H2N-CO-OH carbamic acid (PIN) | H2N-CO– carbamoyl (retained name; preferred prefix) aminocarbonyl |
| H2N-CS-OH carbamothioic O-acid (PIN) | H2N-CS– carbamothioyl (retained name; preferred prefix) aminocarbonothioyl |
| H2N-C(=NH)-OH carbamimidic acid (PIN) | H2N-C(=NH)– carbamimidoyl– (retained name; preferred prefix) C-aminocarbonimidoyl |
| Cl-CO-OH carbonochloridic acid (PIN) | Cl-CO– carbonochloridoyl (preferred prefix) chlorocarbonyl |
| NC-CO-OH carbonocyanidic acid (PIN) | NC-CO– carbonocyanidoyl (preferred prefix) cyanocarbonyl |
| Br-CS-OH carbonobromidothioic O-acid (PIN) | Br-CS– carbonobromidothioyl (preferred prefix) bromocarbonothioyl |
| Cl-C(=NH)-OH carbonochloridimidic acid (PIN) | Cl-C(=NH)– carbonochloridimidoyl (preferred prefix) C-chlorocarbonimidoyl |
| HOO-CO-OH carbonoperoxoic acid (PIN) | HOO-CO– carbonoperoxoyl (preferred prefix) hydroperoxycarbonyl |
P-65.2.1.6 The prefix ‘carboxy’ and prefixes for chalcogen analogues.
The prefix ‘carboxy’ for –COOH is a retained prefix. Chalcogen analogues are named by functional replacement nomenclature provided that it is not necessary to specify the location of the chalcogen atom. Specification of chalcogen atoms is accomplished by compound prefixes formed by concatenation.
Examples:
HS-CO–
sulfanylcarbonyl (preferred prefix)
HS-CS–
dithiocarboxy (preferred prefix)
sulfanylcarbonothioyl
HO-CS–
hydroxycarbonothioyl (preferred prefix)
HOOC-O–
carboxyoxy (preferred prefix)
HOOC-S–
carboxysulfanyl (preferred prefix)
HOOC-NH–
carboxyamino (preferred prefix)
HS-CO-O–
(sulfanylcarbonyl)oxy (preferred prefix)
(1) by using an infix when the position of the chalcogen atoms is not known;Methods (1) or (2) lead to preferred IUPAC names.(2) by compound prefixes formed by concatenation;
(3) by thiohydroperoxy prefixes using the italic prefixes SO- or OS-, as necessary.
Examples:
HOS-CO– or HSO-CO–
(1) carbono(thioperoxoyl) (preferred prefix)
(3) (thiohydroperoxy)carbonyl
HS-O-CO-O–
(2) [(sulfanyloxy)carbonyl]oxy (preferred prefix)
(3) [(SO-thiohydroperoxy)carbonyl]oxy
HSS-CO-O–
(2) (disulfanylcarbonyl)oxy (preferred prefix)
(3) [(dithiohydroperoxy)carbonyl]oxy
Acyl groups derived from carbonic acid and carbonic acids modified by functional replacement are divalent groups with the two free valences belonging to the ‘diyl’ type, such as CO<. When the two free valences are of the ‘ylidene’ type, =C=O for example, names of acyl groups are no longer used to designate such groups; systematic substitutive names are used instead.
Examples:
=C=S
sulfanylidenemethylidene (preferred prefix)
thioxomethylidene
=C=NH
iminomethylidene (preferred prefix)
=C=N-NH2
hydrazinylidenemethylidene (preferred prefix)
diazanylidenemethylidene
Cyanic acid is the retained name for NC-OH. The functional replacement name based on carbonic acid would be carbononitridic acid, but this name has not been used and, although systematic, is only recommended for general nomenclature. Cyanic acid is classified as an acid, thus generating anhydrides (see P-65.7.2) and esters (see P-65.6.3.2).
Preferred prefixes derived from cyanic acid are ‘cyano’ for –CN and ‘cyanato’ for –O-CN, ‘thiocyanato’ for –S-CN, ‘selenocyanato’ for –Se-CN, and ‘tellurocyanato’ for –Te-CN. Functional replacement by –OO–, –S–, –Se–, and –Te– is expressed by the appropriate functional replacement prefix. This exception to the use of infixes in the functional replacement nomenclature applied to the mononuclear inorganic acids (see P-67) is necessary to maintain well entrenched traditional names and their related isocyanates, such as isothiocyanates. Parentheses are used to enclose chalcogen prefixes to avoid the possibility of ambiguity.
Examples:
| NC-SH thiocyanic acid (PIN) carbononitridothioic acid | NC-S– thiocyanato (preferred prefix) carbononitridoylsulfanyl carbononitridoylthio |
| NC-OOH peroxycyanic acid (PIN) carbononitridoperoxoic acid | NC-OO– cyanoperoxy (preferred prefix) carbononitridoylperoxy |
| NC-SS-H dithioperoxycyanic acid (PIN) carbononitrido(dithioperoxoic) acid | NC-SS– cyanodisulfanyl (preferred prefix) carbononitridoyldisulfanyl carbononitridoyldithio |
| NC-CH2-COOH cyanoacetic acid (PIN) carbononitridoylacetic acid | NC-S-CH2-CH2-COOH 3-(thiocyanato)propanoic acid (PIN) 3-(carbononitridothio)propanoic acid |
Di-, tri-, tetra-, and polycarbonic acids belong to the series of homopolynuclear acids, whose central atom is carbon. Their generic formula is HO-[CO-O]n-H, where n is 2, 3, 4, etc. and they are named by adding a multiplying prefix corresponding to the number of carbon atoms to the name of ‘carbonic acid’ or a functional replacement derivative. The structure is numbered consecutively from one end to the other, starting from and ending at a carbon atom:
Examples:
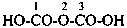
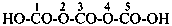
tricarbonic acid (PIN)
P-65.2.3.1.1 General methodologyP-65.2.3.1.1 General methodology
P-65.2.3.1.2 Replacement by –OO–, –S–, =S, –Se–, =Se, –Te–, =Te, –NH–, =NH, and =NHNH2
P-65.2.3.1.3 Replacement by halides and pseudohalides
P-65.2.3.1.4 Replacement by –NH2 and –NHNH2 groups.
P-65.2.3.1.5 Substituent groups derived from di-, tri-, tetra-, and polycarboic acids
Nomenclature for functional analogues of the di-, tri-, tetra-, and polycarbonic acids follows the principles for naming polynuclear inorganic oxo acids (see P-67.2.1). Prefixes are used to indicate functional replacement and the chain is numbered consecutively from one end to the other, starting from and ending at a carbon atom. These prefixes are listed in Table 4.2; they are cited in alphabetical order in front of the retained name of the polyacid, with appropriate locants as required.
P-65.2.3.1.2 Replacement by –OO–, –S–, =S, –Se–, =Se, –Te–, =Te, –NH–, =NH, and =NHNH2
Functional replacement of oxygen atom(s), –OH, =O, –O–, is denoted by prefixes, i.e., peroxy for –OO–; thio for –S– or =S; seleno for –Se– or =Se; telluro for –Te–or =Te; imido for –NH– or =NH, and hydrazono for =NHNH2. The position of each replaced oxygen atom is denoted by the appropriate numerical locant.
P-65.2.3.1.2.1 Superscripted italic letter locants N2, N3, etc. are used to designate substitution on nitrogen atoms that are not amide linkages that are part of the chain for which arabic numbers are used as locants.
Examples:
This is a change. Primed letter locants, N′, N′′, N′′′, etc. were previously used as locants for nitrogen atoms that are not amide linkages that are part of the chain for which arabic numbers are used as locants. 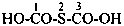
2-thiodicarbonic acid (PIN)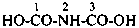
2-imidodicarbonic acid (PIN)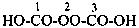
2-peroxydicarbonic acid (PIN)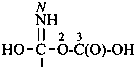
1-imidodicarbonic acid (PIN)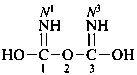
1,3-diimidodicarbonic acid (PIN)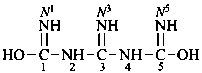
1,2,3,4,5-pentaimidotricarbonic acid (PIN)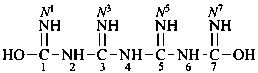
1,2,3,4,5,6,7-heptaimidotetracarbonic acid (PIN)
1-peroxydicarbonic acid (PIN)
1,3-diperoxydicarbonic acid (PIN)
The use of superscripted letter locants is a change from previous practice where numerical locants were placed in front of the letter locants such as 1-O and 3-O, as described in ref. 1, Rule C-213.1.
Examples:
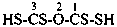
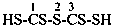
pentathiodicarbonic acid (PIN)
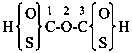
1,3-dithiodicarbonic acid (PIN;
the location of the sulfur atoms is unknown)
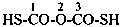
1,3-dithiodicarbonic S1,S3-acid (PIN)
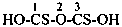
1,3-dithiodicarbonic O1,O3-acid (PIN)
Examples:
H{S/O}C-O-CS-OH [(thiocarboxy)oxy]methanethioic O-acid (PIN)
Prefixes bromo for –Br, chloro for –Cl, fluoro for –F, iodo for –I , azido for –N3, isocyano for –NC, and isocyanato for –NCO (and chalcogen analogues) are used to indicate functional replacement.
Examples:
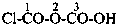
2-imido-1-isocyanatodicarbonic acid
(locants are used to avoid ambiguity)
The prefixes amido and hydrazido are used to indicate functional replacement by –NH2 and –NHNH2 groups, respectively. Italic letter locants N, N′, etc. are used to designate substitution on nitrogen atoms that are not amide linkages for which numerical locants are used.
Examples:
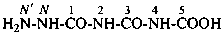
1-hydrazido-2,4-diimidotricarbonic acid (PIN)
Names of substituent groups are formed by substitution or concatenation as required.
Examples:
HS-CS-S-CS–
[(dithiocarboxy)sulfanyl]carbonothioyl (preferred prefix)
[(sulfanylcarbonothioyl)sulfanyl]carbonothioyl
[sulfanyl(thiocarbonyl)sulfanyl](thiocarbonyl)
{not [(dithiocarboxy)sulfanyl]thioformyl}
2-[(carboxyoxy)carbonyl]benzoic acid (PIN)
P-65.3.0 Introduction. The following acids are included in this section:
R-SeO3H selenonic acids
R-TeO3H telluronic acids
R-SO2H sulfinic acids
R-SeO2H seleninic acids
R-TeO2H tellurinic acids
Table 6.2 Suffixes and prefixes used to denote sulfur, selenium, and tellurium acids with chalcogen atoms directly linked to a parent
| Group | Preselected Suffix | Preselected Prefix |
| –SO2-OH | sulfonic acid | sulfo |
| –S(O)-OH | sulfinic acid | sulfino |
| –SeO2-OH | selenonic acid | selenono |
| –Se(O)-OH | seleninic acid | selenino |
| –TeO2-OH | telluronic acid | tellurono |
| –Te(O)-OH | tellurinic acid | tellurino |
Sulfonic, sulfinic, etc., acids are named substitutively by adding an appropriate suffix listed in Table 6.2 to the name of a parent hydride name. Multiplying prefixes ‘di’, ‘tri’, ‘tetra’, etc. are used to denote multiplicity of suffixes. The name ‘sulfanilic acid’ is not retained.
Examples:
butane-2-sulfinic acid (PIN)
4-methylbenzene-1,3-disulfonic acid (PIN)
(not toluene-2,4-disulfonic acid)
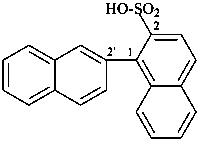
[1,2′-binaphthalene]-2-sulfonic acid (PIN)
4-aminobenzene-1-sulfonic acid (PIN)
(not ‘sulfanilic acid’; this name is not retained)
Oxygen atoms of a suffix acid may be replaced by –OO– and/or other chalcogen analogues, –S– or =S, –Se– or =Se, –Te– or =Te, =NH, and =N-NH2 by functional replacement nomenclature. The general methodology is to modify the suffixes by infixes and use them in systematic substitutive nomenclature in the way prescribed for unmodified suffixes. If necessary, names are formed in accordance with the order of seniority, unmodified acids followed by –OO– > S > Se > Te. This seniority is fully exemplified in Section P-43.
P-65.3.1.2 Peroxy acids
The suffixes given in Table 6.2 are modified by the infix ‘peroxo’ for use in substitutive nomenclature as illustrated by the following suffixes.
–SeO-OOH
seleninoperoxoic acid (preferred suffix)
C6H5-TeO-OOH
benzenetellurinoperoxoic acid (PIN)
Suffixes are modified by the infixes ‘thio’, for –S– or =S, ‘seleno’, for –Se– or =Se, and ‘telluro’, for –Te– or =Te, and used as such. Tautomers are denoted by symbols S, Se, and Te placed in front of the term ‘acid’, to express positions of chalcogen atoms when known. The infixes ‘thioperoxo’, ‘selenoperoxo’, etc. are used to indicate functional replacement in peroxy acids.
–Se(=S)-OH
seleninothioic O-acid (preselected suffix)
–SO2-OSH
sulfono(thioperoxoic) OS-acid (preselected suffix)
–TeO-SeSH
tellurino(selenothioperoxoic) SeS-acid (preselected suffix)
CH3-CH2-S(O)(S)-OH
ethanesulfonothioic O-acid (PIN)
CH3-CH2-Se(=S)-OH
ethaneseleninothioic O-acid (PIN)
Imidic acids and hydrazonic acids derived from sulfonic, sulfinic, etc., acids are named by using suffixes such as ‘sulfinimidic acid’ for –S(=NH)-OH, ‘sulfonohydrazonic acid’ for –S(O)(=NNH2)-OH. The prefix ‘di’ is used to indicate the replacement of two oxygen atoms (=O) in sulfonic acids, for example, ‘sulfonodiimidic acid’ for –S(=NH)2-OH. Suffixes are listed in Table 4.3.
Examples:
CH3-S(=NH)-OH
methanesulfinimidic acid (PIN)
C6H5-Se(=NH)2-OH
benzeneselenonodiimidic acid (PIN)
benzenesulfonohydrazonic acid (PIN)
naphthalene-2-selenonohydrazonimidothioic acid (PIN)
Hydroximic acids and hydroxamic acids derived from sulfonic, sulfinic, etc. acids are named as N-hydroxysulfonimidic acids and N-hydroxysulfonamides, etc. (see P-66.1.1.3.2), respectively.
Examples:
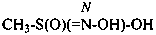
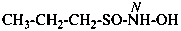
N-hydroxypropane-1-sulfinamide (PIN)
P-65.3.2.1 When another group is also present that has seniority for citation as principal group (see P-41, P-42, P-43), or when all groups cannot be expressed as suffixes, organic oxoacids of sulfur, selenium or tellurium are named by adding to the name of the parent compound the appropriate prefix given in Table 6.2. These prefixes can be modified by prefixes designating chalcogen atoms in functional replacement nomenclature when the position of the chalcogen atom is not known or when it is not desirable to indicate such position.
Examples:
4-sulfobenzene-1,2-dicarboxylic acid (PIN)
4-sulfophthalic acid
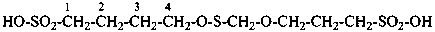
4-({[(3-sulfopropoxy)methyl]sulfanyl}oxy)butane-1-sulfonic acid (PIN)
HO-SO-CH2-COOH
sulfinoacetic acid (PIN)
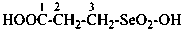
3-selenonopropanoic acid (PIN)
H{S/O}S-CH2-CH2-SO2-OH
2-(thiosulfino)ethane-1-sulfonic acid
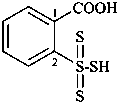
2-(trithiosulfo)benzoic acid (PIN)
2-(sulfanylsulfonodithioyl)benzoic acid
P-65.3.2.2.1 Acyl sulfonic, sulfinic, selenonic, seleninic, telluronic, and tellurinic groups, R-EOx–, –OxE-R-EOx– or –OxE-R-[R′-EOx-]-R′′-EOx–, where E = S, Se, or Te, x = 1 or 2, and R, R′, and R′′ are chains, rings, or ring systems; their functional replacement analogues are groups derived by the removal of the hydroxy group from each sulfonic, sulfinic, or related selenium or tellurium acid group that is expressed as the principal characteristic group by an appropriate suffix.
P-65.3.2.2.2 Names for acyl groups derived from sulfonic and sulfinic acids, and their Se and Te counterparts, by removal of the -OH group from each sulfonic, sulfinic, etc. acid expressed as a suffix are formed by changing the ‘ic acid’ ending of the suffix to ‘yl’. When the suffix is modified by functional replacement nomenclature, the ending of the corresponding acyl group is ‘oyl’. Acyl groups formed by concatenation, for example, phenylsulfonyl, may be used in general nomenclature.
The formation of simple substitutive preferred acyl prefixes directly from the name of the sulfonic acid, sulfinic acid, etc., as in ‘benzenesulfonyl’, instead of the traditional method of concatenation as in ‘phenylsulfonyl’ is a distinct change to simplify names. To facilitate name interpretation, the preferred prefixes are enclosed in parentheses even though they are simple prefixes (P-16.5.1.4).
Examples:
CH3-Se(O)–
methaneseleninyl (preferred prefix)
methylseleninyl
CH3-CH2-S(O)(S)–
ethanesulfonothioyl (preferred prefix)
ethylsulfonothioyl
C6H5-S(Se)–
benzenesulfinoselenoyl (preferred prefix)
phenylsulfinoselenoyl
CH3-CH2-S(=NH)–
ethanesulfinimidoyl (preferred prefix)
ethylsulfinimidoyl
When the name of an acyl group cannot be derived directly from that of the acid expressed by a suffix, a concatenation procedure is used. For this procedure names of divalent mononuclear acyl groups are required. Acyl groups corresponding to sulfuric and sulfurous acids and the corresponding selenium and tellurium acids are formed from the acids by subtracting all -OH groups from the parent acid. The names used in the nomenclature of organic compounds are as follows:
–SO–
sulfinyl (preselected prefix)
thionyl
–SeO2–
selenonyl (preselected prefix)
–SeO–
seleninyl (preselected prefix)
–TeO2–
telluronyl (preselected prefix)
–TeO–
tellurinyl ( preselected prefix)
Examples:
–S(=S)(=S)–
sulfonodithioyl (preselected prefix)
–S(=NH)–
sulfinimidoyl (preselected prefix)
–Se(=O)(=NNH2)–
selenonohydrazonoyl (preselected prefix)
–Se(=S)(=NH)–
selenonimidothioyl (preselected prefix)
Examples:
Cl-S(O)–
chlorosulfinyl (preselected prefix)
H2N-SO2–
sulfamoyl (preselected prefix)
aminosulfonyl
H-SO–
hydrosulfinyl (preselected prefix)
CH3-CO-O-SO2–
(acetyloxy)sulfonyl (preferred prefix)
acetoxysulfonyl
CH3-O-S(=NH)–
S-methoxysulfinimidoyl (preferred prefix)
HO-SO2-O–
sulfooxy (preselected prefix)
H-SeO2–
hydroselenonyl (preselected prefix)
–S-SO2-S–
sulfonylbis(sulfanediyl) (preselected prefix)
–O-SO-O–
sulfinobis(oxy) (preselected prefix)
Polyfunctional compounds are named in accordance with the general order of seniority of suffixes described in Sections P-41 and P-43. When required, numbering is based on the seniority order described in P-61.1.
Examples:
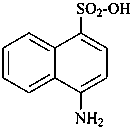
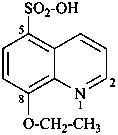
8-ethoxyquinoline-5-sulfonic acid (PIN)
8-hydroxy-5,7-dinitronaphthalene-2-sulfonic acid (PIN)
7-aminonaphthalene-1,3-disulfonic acid (PIN)
2-(trithiosulfo)benzene-1-sulfonothioic S-acid (PIN)
P-65.4.1 General methodology
Acyl group names that are described in preceding sections are used unchanged to denote substituent groups. Thus, the traditional way of using acyl groups derived from acyclic carboxylic acids to name ketones, pseudoketones, and heterones is maintained (see P-65.1.7 for more examples).
Examples:
2-(methanesulfonyl)benzoic acid (PIN)
2-(methylsulfonyl)benzoic acid
2-(cyclohexanecarbonyl)naphthalene-1-carboxylic acid (PIN)
2-(cyclohexylcarbonyl)naphthalene-1-carboxylic acid
[1-(4-chlorobenzoyl)-5-methoxy-2-methyl-1H-inden-3-yl]acetic acid (PIN)
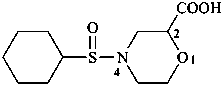
4-(cyclohexanesulfinyl)morpholine-2-carboxylic acid (PIN)
4-(cyclohexylsulfinyl)morpholine-2-carboxylic acid
(propane-1-sulfonyl)benzene (PIN)
(propylsulfonyl)benzene
[2,3-dichloro-4-(2-methylidenebutanoyl)phenoxy]acetic acid (PIN)
P-65.5.1 Acyl halides from suffix acidsP-65.5.1 Acyl halides from suffix acids
P-65.5.2 Acyl pseudohalides from suffix acids
P-65.5.3 Acyl halides and pseudohalides from carbonic, cyanic, and polycarbonic acids
P-65.5.4 Acyl halides and pseudohalides as substituent groups
Acyl halides in which hydroxy groups of all acid groups expressed as the suffix denoting the principal characteristic group (carboxylic, sulfonic, sulfinic, selenonic, etc. acids) have been replaced by halogen atoms (F, Cl, Br, and I) are named by citing the name of the acyl group (see P-65.1.7) followed by the name(s) of the specific class(es) as a separate word(s), in alphabetical order, each preceded by a multiplicative prefix, as needed.
| Halide | Prefix | Pseudohalide | Prefix | ||
| –F | fluoride | fluoro | –N3 | azide | azido |
| –Cl | chloride | chloro | –CN | cyanide | cyano |
| –Br | bromide | bromo | –NC | isocyanide | isocyano |
| –I | iodide | iodo | –NCO | isocyanate | isocyanato |
| –NCS | isothiocyanate | isothiocyanato | |||
| –NCSe | isoselenocyanate | isoselenocyanato | |||
| –NCTe | isotellurocyanate | isotellurocyanato | |||
Examples:
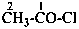
HCO-Br
formyl bromide (PIN)
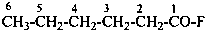
hexanoyl fluoride (PIN)
cyclohexanecarboximidoyl chloride (PIN)
benzenesulfinyl chloride (PIN)
cyclohexanecarbothioyl chloride (PIN)
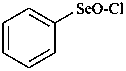
benzeneseleninyl chloride (PIN)
Cl-CO-CH2-CO-Cl
propanedioyl dichloride (PIN)
malonyl dichloride
Cl-CO-CO-Cl
oxalyl dichloride (PIN)
ethanedioyl dichloride
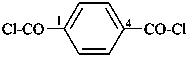
benzene-1,4-dicarbonyl dichloride (PIN)
terephthaloyl dichloride
Br-O2S-CH2-CH2-SO2-Br
ethane-1,2-disulfonyl dibromide (PIN)
Br-CO-CH2-CH2-CO-Cl
butanedioyl bromide chloride (PIN)
succinyl bromide chloride
H2N-CO-CO-Br
oxamoyl bromide (PIN)
P-65.5.2.1 Acyl pseudohalides in which hydroxy groups of all acid groups expressed as the suffix denoting the principal characteristic group (carboxylic, sulfonic, sulfinic, selenonic, etc. acids) have been replaced by pseudohalogen groups (N3, CN, NC, NCO, NCS, NCSe, NCTe) are named by citing the name of the acyl group (see P-65.1.7) followed by the name(s) of the class(es) as separate words, preceded by a multiplicative prefix, as needed. When a choice has to be made, the senior pseudohalide group is chosen in accordance with the decreasing order of seniority: N3 > CN > NC > NCO > NCS > NCSe > NCTe. Halogen atoms are senior to pseudohalogen groups.
The names formyl, acetyl, benzoyl, oxalyl, and oxamoyl are retained to generate preferred IUPAC names.
Examples:
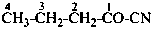
SCN-CO-CO-NCS
oxalyl diisothiocyanate (PIN)
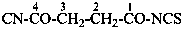
butanedioyl isocyanide isothiocyanate (PIN)
P-65.5.3.1 Acyl groups derived from carbonic acid, carbamic acid, and related acids, such as ‘carbonyl’ from carbonic acid, ‘carbamoyl’ from carbamic acid, and ‘carbamimidoyl’ from carbamimidic acid are used to form the names of the corresponding acyl halides.
Examples:
Br-CO-Cl
carbonyl bromide chloride (PIN)
(not carbonobromidic chloride)
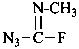
N-methylcarbonazidimidoyl fluoride (PIN)
NC-CO-Cl
carbonocyanidoyl chloride (PIN)
H2N-CO-NCO
carbamoyl isocyanate (PIN)
Examples:
Cl-CO-O-CO-Br
dicarbonic bromide chloride (PIN)
2-imidodicarbonic dichloride (PIN)
OCN-CO-O-CO-NCO
dicarbonic diisocyanate (PIN)
1,2-diimido-4,5-dithiotricarbonic 5-bromide 1-chloride (PIN)
(1) as acyl halides or pseudohalides of carbononitridic acid;Method (1) generates preferred IUPAC names.(2) by citing the name of the halide or pseudohalide after the name of the acid.
Examples:
NC-N3
carbononitridic azide (PIN)
cyanic azide
When another group is present that has priority for citation as principal group or when attached to another substituting group, an acyl halide or pseudohalide is expressed:
(1) by a prefix formed from the name of the acid, for example, ‘carbonochloridoyl’;Method (1) leads to preferred IUPAC names when the suffix ‘-carboxylic acid’ is used to name the corresponding acid; method (3) generates preferred IUPAC names for acyclic carbon chains.(2) by a compound prefix composed of a halo or halogeno prefix and an appropriate divalent
acyl group, such as ‘sulfonyl’, for example, fluorosulfonyl;
(3) at the end of an acyclic carbon chain by a prefix denoting the halide or pseudohalide group and the prefix ‘oxo’, or a chalcogen analogue of oxo, such as sulfanylidene.
Seniority for numbering follows that for acids, for which see P-65.1.2.3. For seniority of halides and pseudohalides, see P-65.5.2.1.
Examples:
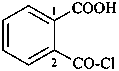
(1) 2-carbonochloridoylbenzoic acid (PIN)
(2) 2-(chlorocarbonyl)benzoic acid
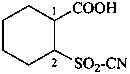
(1) 2-(cyanosulfonyl)cyclohexane-1-carboxylic acid (PIN)
(2) 2-sulfurocyanidoylcyclohexane-1-carboxylic acid
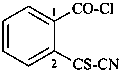
(1) 2-carbonocyanidothioylbenzoyl chloride (PIN)
(2) 2-(cyanocarbonothioyl)benzoyl chloride
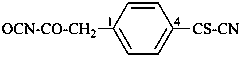
(4-carbonocyanidothioylphenyl)acetyl isocyanate (PIN)
[not 4-(2-isocyanato-2-oxoethyl)benzenecarbothioyl cyanide,
acetyl is senior to carbothioyl]
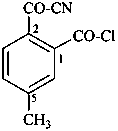
2-carbonocyanidoyl-5-methylbenzoyl chloride (PIN)
2-(cyanocarbonyl)-5-methylbenzoyl chloride
Br-CO-CO-CH2-COOH
(3) 4-bromo-3,4-dioxobutanoic acid (PIN)
Br-CO-O-CO-CH2-COOH
(1) 3-(carbonobromidoyloxy)-3-oxopropanoic acid (PIN)
P-65.6.1 General methodologyP-65.6.1 General methodology
P-65.6.2 Salts
P-65.6.3 Esters, lactones, and related compounds
Neutral salts and esters are both named using the name of the anion derived from the name of the acid. Anion names are formed by changing an ‘-ic acid’ ending of an acid name to ‘-ate’ and an ‘-ous acid’ ending of an acid name to ‘-ite’. Then, salts are named using the names of cations, and esters the names of organyl groups, cited as separate words in front of the name of the anion.
P-65.6.2 Salts
P-65.6.2.1 Neutral salts of acids are named by citing the name of the cation(s) followed by the name of the anion (see P-72.2.2.2) as a separate word. Different cations are cited in alphabetical order. Formation of salts is a functionalization and not a substitution. Thus, all retained names, both those used as preferred IUPAC names and those used only for general nomenclature, can be used without restriction. This rule applies equally to acids expressed by suffixes and carbonic, cyanic, oxalic, and polycarbonic acids.
Examples:
CH3-CH2-CS-S– Na+
sodium propane(dithioate) (PIN)
(CH3-COO–)2 Ca2+
calcium diacetate (PIN)
C6H5-SO-O– Na+
sodium benzenesulfinate (PIN)
K+ –OOC-CH2-CH2-COO– Na+
potassium sodium butanedioate (PIN)
potassium sodium succinate
NH4+ –OOC-CH2-CH2-CH2-CH2-COO– K+
ammonium potassium hexanedioate (PIN)
ammonium potassium adipate
C(O)O22– 2Na+
disodium carbonate (PIN)
(CH3-COO–)4 Ge4+
germanium tetraacetate (PIN)
Example:
P-65.6.2.3.1 Acid salts of polybasic organic acids are named in two ways:
(1) by substitutive nomenclature in which the free acid is cited as a prefix to the name of the anion;Method (1) generates preferred IUPAC names, except when the structure of the acid salt is unknown. Anionic substituents, such as –COO–, –SO3–, –SO2– are described by the prefix names ‘carboxylato’, ‘sulfonato’, and ‘sulfinato’, respectively, and similarly for the corresponding selenium and tellurium acids.(2) in the same way as the neutral salts, the remaining acid hydrogen atom(s) being indicated by the word ‘hydrogen’ (preceded by a numerical prefix, ‘di’, ‘tri’, etc., as appropriate) inserted as a separate word between the name(s) of the cation(s) and the name of the anion. When required, cations are cited in names in alphabetical order.
Examples:
HOOC-CH2-CH2-COO– NH4+
(1) ammonium 3-carboxypropanoate (PIN)
(2) ammonium hydrogen butanedioate
ammonium hydrogen succinate
(2) sodium hydrogen 2-(carboxylatomethyl)benzoate (PIN)
(2) potassium sodium hydrogen propane-1,2,3-tricarboxylate (PIN)
(HOOC-CH2-CH2-COO–)3 Sb3+
(1) antimony tris(3-carboxypropanoate) (PIN)
(2) antimony tris(hydrogen butanedioate)
Examples:
CH3-P(O)(O–)2 K+ H+
potassium hydrogen methylphosphonate (PIN)
Example:
P-65.6.3.1 DefinitionsP-65.6.3.1 Definitions
P-65.6.3.2 General methodology
P-65.6.3.3 Peferred IUPAC names for esters
P-65.6.3.4 Pseudoesters
P-65.6.3.5 Cyclic esters
P-65.6.3.6 Acylals
P-65.6.3.1.1 Esters of organic oxoacids, R-C(O)-O-R′ (R can be H) or R-S(O)x-O-R (R ≠ H) or chalcogen analogues, are compounds formally derived from an organic oxoacid R-C(O)-OH (R can be H) or R-S(O)x-OH (R ≠ H) and an alcohol, phenol, heterol, or enol by a formal loss of water from an acidic hydroxy group of the former and a hydroxy group of the latter. By extension, they are ‘acyl’ derivatives of alcohols, etc. Esters derived from chalcogen analogs of organic oxoacids and chalcogen analogues of alcohols (thiols, selenols, tellurols), phenols, heterols, and enols, i.e., acyl derivatives of chalcogen analogues of alcohols (thiols, selenols, tellurols), phenols, heterols, and enols, are also included.
For esters derived from inorganic oxoacids, see Section P-67.
P-65.6.3.1.2 Pseudoesters are compounds having the generic formula R-E(=O)x(OZ) and chalcogen analogues where x = 1 or 2 and Z is not a carbon atom but an element from the following list: B, Al, In, Ga, Tl, Si, Ge, Sn, Pb, N(cyclic), P, As, Sb, Bi. Pseudoesters are ranked as esters in the seniority order of classes (see P-41).
Examples:
CH3-CH2-SO2-S-Ge(CH3)3
S-(trimethylgermyl) ethanesulfonothioate (PIN)
P-65.6.3.2.1 All preferred IUPAC names for esters are named by functional class nomenclature.
Examples:
CH3-O-CO-CH2-CH2-CO-O-CH2-CH3
ethyl methyl butanedioate (PIN)
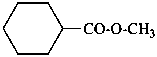
methyl cyclohexanecarboxylate (PIN)
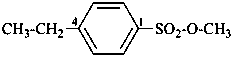
methyl 4-ethylbenzene-1-sulfonate (PIN)
In functional class nomenclature for esters the multiplicative operation (P-13.6.2) is used to name assemblies of identical parent anionic components linked by a di- or multivalent ‘hydroxylic’ component.
A change occurs in the multiplicative operation applied to esters from previous recommendations. The bi- or polyvalent functional class name is cited as the organyl (alkanediyl, arylene, etc.) group cited immediately before the name of the acid component denoted by the anion name derived from the appropriate acid (see P-72.2.2.2.1) rather than alphabetically along with other monovalent organyl groups as was done in earlier recommendations.
Examples:
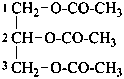
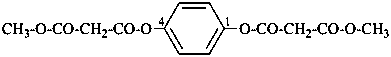
dimethyl 1,4-phenylene dipropanedioate (PIN)
1,4-phenylene di(methyl propanedioate)
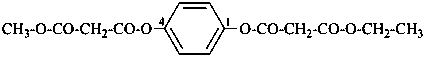
ethyl methyl 1,4-phenylene dipropanedioate (PIN)
When, in an ester with the general structure R-CO-O-R′ or R-S(O)x-O-R′, another group is present that has priority for citation as the principal group or when all ester groups cannot be described by the methods prescribed for naming esters, an ester group is indicated by prefixes as ‘acyloxy’ for the group R-CO-O–, and ‘alkoxy...oxo’, ‘(alkyloxy)...oxo’, ‘(alkanyloxy)...oxo’, ‘alkoxycarbonyl’, ‘(alkyloxy)carbonyl’ or ‘(alkanyloxy)carbonyl’ for the group –CO-OR′.
The systematic name ‘acetyloxy’ is preferred to the contracted name ‘acetoxy’ that may be used in general nomenclature.
Seniority for numbering follows that for acids, for which see P-65.1.2.3.
Examples:
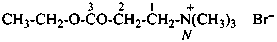
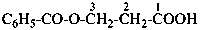
3-(benzoyloxy)propanoic acid (PIN)
3-[(phenylcarbonyl)oxy]propanoic acid
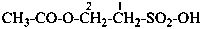
2-(acetyloxy)ethane-1-sulfonic acid (PIN)
2-acetoxyethanesulfonic acid
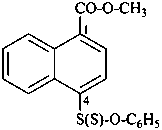
methyl 4-(phenoxysulfinothioyl)naphthalene-1-carboxylate (PIN)
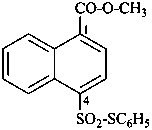
methyl 4-[(phenylsulfanyl)sulfonyl]naphthalene-1-carboxylate (PIN)
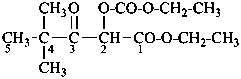
ethyl 2-[(ethoxycarbonyl)oxy]-4,4-dimethyl-3-oxopentanoate (PIN)
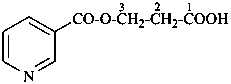
3-[(pyridine-3-carbonyl)oxy]propanoic acid (PIN)
3-(nicotinoyloxy)propanoic acid
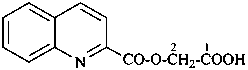
[(quinoline-2-carbonyl)oxy]acetic acid (PIN)
[(quinolin-2-ylcarbonyl)oxy]acetic acid;
(see P-65.4.1 for naming acyl groups derived from acids)
P-65.6.3.3.1 MonoestersP-65.6.3.3.1 Monoesters
P-65.6.3.3.2 Polyesters derived from a single acid component
P-65.6.3.3.3 Polyesters formed from a single ‘alcoholic’ component
P-65.6.3.3.4 Polyesters derived from multiple acid components and multiple ‘alcoholic’ components
P-65.6.3.3.5 Partial esters from polybasic acids and their salts
P-65.6.3.3.6 Substitutive nomenclature is senior to functional class nomenclature for preferred IUPAC names for esters
P-65.6.3.3.7 Esters of acids modified by functional replacement nomenclature
Monoesters formed from a monobasic acid and a ‘monohydroxylic’ component are named systematically by placing the ‘hydroxylic’ component denoted by an organyl group (alkyl, aryl, etc.) in front of the name of the acid component expressed as an anion derived from the appropriate acid (see P-72.2.2.2.1).
Examples:
CH3-[CH2]6-CO-O-C(CH3)3
tert-butyl octanoate (PIN)
1,1-dimethylethyl octanoate
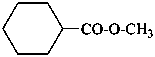
methyl cyclohexanecarboxylate (PIN)
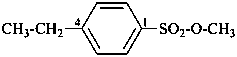
methyl 4-ethylbenzene-1-sulfonate (PIN)
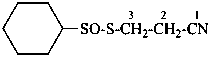
S-(2-cyanoethyl) cyclohexanesulfinothioate (PIN)
{not 3-[(cyclohexanesulfinyl)sulfanyl]propanenitrile
nor 3-[(cyclohexylsulfinyl)sulfanyl]propanenitrile;
see P-65.4.1 for naming acyl groups derived from acids}
P-65.6.3.3.2.1 Fully esterified acids derived from a single acid are systematically named by placing the name(s) of the hydroxylic component denoted by an organyl group(s) (alkyl, aryl, etc.) as separate word(s) in front of the name of the acid component denoted by the anion name derived from the appropriate acid (see P-72.2.2.2.1). Multiplicative prefixes are used to denote a multiplicity of identical organyl groups; different organyl groups are cited in alphanumerical order (see P-14.5). When necessary, locants are cited at the front of the organyl groups.
This rule applies equally to carboxylic, sulfonic, sulfinic, etc. acids.
Examples:
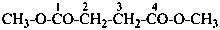
CH3-CH2-O-CO-CH2-CO-O-CH3
ethyl methyl propanedioate (PIN)
ethyl methyl malonate
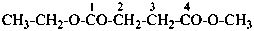
ethyl methyl butanedioate (PIN)
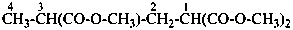
trimethyl butane-1,1,3-tricarboxylate (PIN)
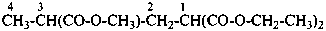
1,1-diethyl 3-methyl butane-1,1,3-tricarboxylate (PIN)
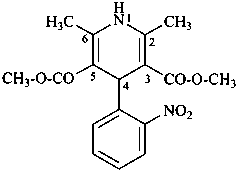
dimethyl 2,6-dimethyl-4-(2-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate (PIN)
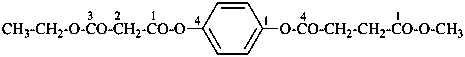
4-[(3-ethoxy-3-oxopropanoyl)oxy]phenyl methyl butanedioate (PIN)
(not ethyl 4-[(4-methoxy-4-oxobutanoyl)oxy]phenyl propanedioate;
butanedioate is preferred to propandioate)
P-65.6.3.3.2.2.1 Esters of acid components whose preferred IUPAC names are derived using multiplicative nomenclature are named by two methods:
(1) all organyl components representing the ‘hydroxylic’ components are cited in front of the name of the multiplied acid component;Method (1) leads to preferred IUPAC names.(2) for esters where both organyl components representing the ‘hydroxylic’ component are exactly the same are cited with the acid component preceded by a numerical term ‘bis-’, ‘tris-’, etc.
Examples:
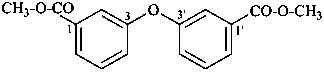
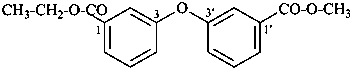
(1) ethyl methyl 3,3′-oxydibenzoate (PIN)
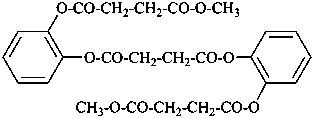
(1) dimethyl butanedioylbis(oxy-2,1-phenylene) dibutanedioate (PIN)
(not bis{2-[(4-methoxy-4-oxobutanoyl)oxy]phenyl} butanedioate;
the PIN expresses two parent dicarboxylic acids)
(2) butanedioylbis(oxy-2,1-phenylene) bis(methyl butanedioate)
Example:
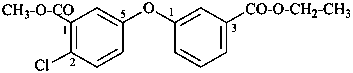
Esters derived from a single ‘polyhydroxylic’ component are named by placing the name of the ‘polyhydroxylic’ component denoted by a multivalent organyl group (alkyl, aryl, etc.) in front of the name(s) of the acid component denoted by the anion names derived from the appropriate acid(s) (see P-72.2.2.2.1).
P-65.6.3.3.3.1 When anions are identical functional class multiplicative nomenclature is used. Names are formed by citing the multivalent group, the multiplicative prefix, and the multiplied anionic component name. Multiplicative prefixes ‘di’, ‘tri’, etc. are used when anions are unsubstituted; when substituted, prefixes ‘bis’, ‘tris’, etc. are used.
Examples:
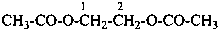
ClCH2-CO-O-CH2-CH2-CH2-O-CO-CH2Cl
propane-1,3-diyl bis(chloroacetate) (PIN)
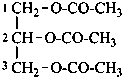
propane-1,2,3-triyl triacetate (PIN)
1,2-phenylenedi(propan-3,1-yl) diacetate [PIN;
see P-15.3.0 (2) and P-51.3.1]
(1) names of anions are cited in alphanumerical order preceded by a locant, when required; multiplicative prefixes are used to denote a multiplicity of identical anionic components;Method (1) generates preferred IUPAC names but names formed by using method (2) are acceptable in general nomenclature.(2) one anion is chosen as principal anion and all other ester groups are expressed as prefixes in the name of the organyl group. The seniority order of anions is corresponding to that of acids (see seniority order of acids in P-41).
Examples:
(1) 1,4-phenylene acetate dichloroacetate (PIN)
(2) 4-(acetyloxy)phenyl dichloroacetate
(1) propane-1,2,3-triyl 1,2-diacetate 3-propanoate (PIN)
(2) 2,3-bis(acetyloxy)propyl propanoate
(1) propane-1,2,3-triyl 2-acetate 1-hexadecanoate 3-[(9Z)-octadec-9-enoate] (PIN)
(2) 2-(acetyloxy)-3-(hexadecanoyloxy)propyl (9Z)-octadec-9-enoate
Multiplicative nomenclature, skeletal replacement (‘a’) nomenclature, or phane nomenclature is used when specific conditions for their use exist and are fulfilled.
P-65.6.3.3.4.1 Polyester names formed by using functional class multiplicative nomenclature
Symmetrical esters are named by including the organyl constituent in the multiplied anion component name. When this condition is not fulfilled, in unsymmetrical esters, the organyl constituents are cited at the beginning of the name, in alphanumerical order.
Example:
Polyesters that cannot be named by functional class multiplicative nomenclature as described above, are named by using substitutive nomenclature to generate the names of the organyl substituents and those of the anions. When required, seniority of rings, ring systems, and chains; number and location of substituents; and alphanumerical order is applied in forming the alcoholic component of a functional class ester name (see P-41 through P-45):
Examples:
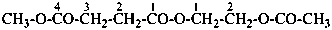
2-(acetyloxy)ethyl methyl ethane-1,2-diyl dibutanedioate (PIN)
bis[2-(acetyloxy)ethyl] propanedioate (PIN)
propanedioylbis(oxyethane-2,1-diyl) diacetate
2-(acetyloxy)ethyl 2-(propanoyloxy)ethyl butanedioate (PIN)
butanedioylbis(oxyethane-2,1-diyl) acetate propanoate
phenyl 3-(benzoyloxy)benzoate (PIN)
[not 3-(phenoxycarbonyl)phenyl benzoate;
the substituted benzoic acid is senior to the unsubstituted one; see P-45.2.1]
2-[3-(formyloxy)propyl]cyclohexane-1,1-diyldi(propane-3,1-diyl) diacetate (PIN)
4-[(3-ethoxy-3-oxopropanoyl)oxy]phenyl methyl butanedioate (PIN)
(not ethyl 4-[(4-methoxy-4-oxobutanoyl)oxy]phenyl propanedioate;
butanedioic acid is preferred to propanedioic acid
3-(methoxycarbonyl)phenyl 2-methoxy-2-oxoethyl butanedioate (PIN)
[not 2-methoxy-2-oxoethyl 3-(methoxycarbonyl)phenyl butanedioate;
3-(methoxycarbonyl)phenyl is alphabetically before 2-methoxy-2-oxoethyl; see P-14.5]
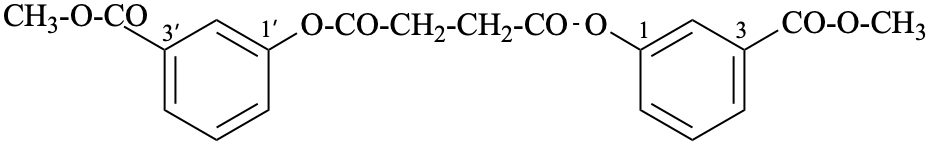
bis[3-(methoxycarbonyl)phenyl] butanedioate (PIN)
(not dimethyl 3,3′-[butanedioylbis(oxy)]dibenzoate;
a dicarboxylic acid is senior to two monocarboxylic acids)
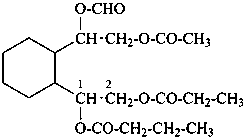
1-{2-[2-(acetyloxy)-1-(formyloxy)ethyl]cyclohexyl}ethane-1,2-diyl 1-butanoate 2-propanoate (PIN)
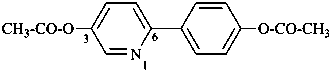
6-[4-(acetyloxy)phenyl]pyridin-3-yl acetate (PIN)
(not 4-[5-(acetyloxy)pyridin-2-yl]phenyl acetate;
the nitrogenous ring is senior to the carbocyclic ring)
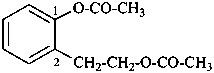
2-[2-(acetyloxy)ethyl]phenyl acetate (PIN)
(not 2-[2-(acetyloxy)phenyl]ethyl acetate;
the ring is senior to the chain)
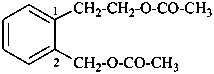
2-{2-[(acetyloxy)methyl]phenyl}ethyl acetate (PIN)
(not 2-{2-[(acetyloxy)ethyl]phenyl}methyl acetate;
the ethyl chain is senior to the methyl chain)
Examples:
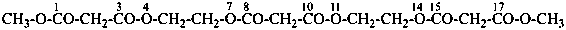
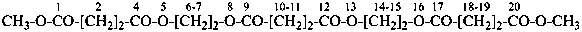
dimethyl 4,9,12,17-tetraoxo-5,8,13,16-tetraoxaicosane-1,20-dioate (PIN)
[a functional class name in which the anion segment is named
by skeletal replacement (‘a’) name]
dimethyl ethane-1,2-diylbis(carbonyloxyethane-2,1-diyl) dibutanedioate (a functional class multiplicative name)
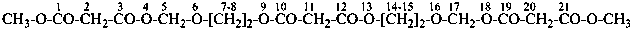
dimethyl 3,10,12,19-tetraoxo-4,6,9,13,16,18-hexaoxahenicosane-1,21-dioate (PIN)
dimethyl 6,8-dioxo-2,5,9,12-tetraoxatridecane-1,13-diyl dipropanedioate
[a functional class name in which the multiplying segment is named by skeletal replacement (‘a’) nomenclature]
dimethyl propanedioylbis(oxyethane-2,1-diyloxymethylene) dipropanedioate (a functional class multiplicative name)
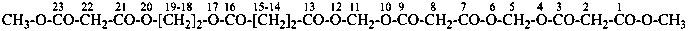
dimethyl 3,7,9,13,16,21-hexaoxo-4,6,10,12,17,20-hexaoxatricosane-1,23-dioate (PIN)
2-{[(methoxycarbonyl)acetyl]oxy}ethyl 3,5,9,11-tetraoxo-2,6,8,12-tetraoxatridecan-1-yl butanedioate
[a functional class name in which one alcoholic segment is named by skeletal replacement (‘a’) nomenclature]
Examples:
dimethyl 2,7,9,14,16,21-hexaoxo-3,6,10,13,17,20-hexaoxa-1,22(1),8,15(1,3)-tetrabenzenadocosaphane-13,223-dicarboxylate (PIN)
(1) substitutively on the basis of the anion, the free acid group(s) and the ester group(s) being cited as prefixes;Method (1) generates preferred IUPAC names.(2) by the procedure for neutral esters and acid salts; the components present are cited in the order, cation, hydrocarbyl group, hydrogen, anion. Numerical locants and italic element symbols (see P-65.1.5.1) are added as necessary to provide specificity. The numbering of the polybasic acid is retained when the hydrogen method is applied to retained names.
Examples:
(1) lithium 4-(ethylsulfanyl)-4-oxobutanethioate (PIN)
(2) lithium S-ethyl butanebis(thioate)
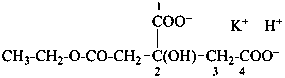
(1) potassium hydrogen 2-(2-ethoxy-2-oxoethyl)-2-hydroxybutanedioate (PIN)
(2) potassium 3-ethyl hydrogen 2-hydroxypropane-1,2,3-tricarboxylate
(2) potassium 3-ethyl hydrogen citrate
(the anionic –OOC– group is preferred to the ester group CH3-CH2-O-CO–)
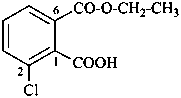
(1) 2-chloro-6-(ethoxycarbonyl)benzoic acid (PIN)
(2) 1-ethyl hydrogen 3-chlorobenzene-1,2-dicarboxylate
(2) 1-ethyl hydrogen 3-chlorophthalate
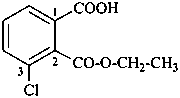
(1) 3-chloro-2-(ethoxycarbonyl)benzoic acid (PIN)
(2) 2-ethyl hydrogen 3-chlorobenzene-1,2-dicarboxylate
(2) 2-ethyl hydrogen 3-chlorophthalate
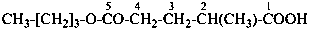
(1) 5-butoxy-2-methyl-5-oxopentanoic acid (PIN)
(2) 5-butyl hydrogen 2-methylpentanedioate
(1) 2-(acetyloxy)-5-butoxy-2-methyl-5-oxopentanoic acid (PIN)
(2) 5-butyl hydrogen 2-(acetyloxy)-2-methylpentanedioate
Examples:
5α-cholestane-3β,6α-diyl diacetate
5α-cholestane-3β,6α-diol diacetate
P-65.6.3.3.7.1 With the exception of retained names, polycarbonic acids and cyanic acid that are described in P-65.6.3.3.7.2, names of esters are all derived from acids modified by functional replacement whose substitutive names are systematically formed, as indicated in sections P-65.1.3 through P-65.1.7.
Structural specification for esters of thio-, seleno- or tellurocarboxylic acids, thio-, seleno-, or tellurosulfonic acids and sulfinic acids and their peroxy analogues is provided by the appropriate italic element symbol, such as S, O, or SO, prefixed to the name of the organyl group.
Examples:
CH3-[CH2]4-CSe-O-CH2-CH3
O-ethyl hexaneselenoate (PIN)
CH3-C(=NH)-O-CH3
methyl ethanimidate (PIN)
methyl acetimidate
CH3-CH2-C(=N-NH2)-O-C2H5
ethyl propanehydrazonate (PIN)
ethyl propionohydrazonate
C6H5-C(=NH)-S-CH3
methyl benzenecarboximidothioate (PIN)
C6H5-C(=N-SH)-S-CH2-CH3
ethyl N-sulfanylbenzenecarboximidothioate (PIN)
C6H5-CO-S-O-CH3
SO-methyl benzene(carbothioperoxoate) (PIN)
CH3-CH2-SO2-O-S-C2H5
OS-ethyl ethanesulfono(thioperoxoate) (PIN)
CH3-S-CO-CO-S-CH3
S,S-dimethyl ethanebis(thioate) (PIN)
Examples:
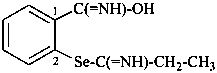
2-(propanimidoylselanyl)benzene-1-carboximidic acid (PIN)
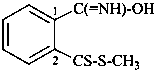
2-[(methylsulfanyl)carbonothioyl]benzene-1-carboximidic acid (PIN)
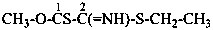
O-methyl (ethylsulfanyl)(imino)ethanethioate (PIN)
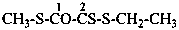
S-methyl (ethylsulfanyl)(sulfanylidene)ethanethioate (PIN)
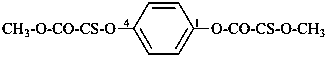
4-{[methoxy(oxo)ethanethioyl]oxy}phenyl methoxy(sulfanylidene)acetate (PIN)
[not O-methyl (4-{[methoxy(oxo)ethanethioyl]oxy}phenoxy)(oxo)ethanethioate;
a carboxylic acid is preferred to a thiocarboxylic acid;
not methyl (4-{[methoxy(sulfanylidene)acetyl]oxy}phenoxy)(sulfanylidene)acetate;
the PIN is lower in alphanumerical order)
P-65.6.3.3.7.2.1 Names of acids modified by functional replacement are used to generate preferred IUPAC names of corresponding esters. Element symbols O, S, etc. and locants are used to designate the location of organyl groups.
Examples:
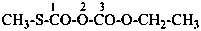
CH3-S-CS-O-CH3
O,S-dimethyl carbonodithioate (PIN)
(CH3)2CH-S-CN
propan-2-yl thiocyanate (PIN)
Examples:
NC-S-CH2-CH2-CO-S-CH2-CH3
S-ethyl 3-(thiocyanato)propanethioate (PIN)
HS-CO-O-CS-O-CH2-CH2-COOH
3-({[(sulfanylcarbonyl)oxy]carbonothioyl}oxy)propanoic acid (PIN)
Compounds having the generic formula R-CO-O-E, where E is not a carbon atom nor an acyl group belong to this class (see P-65.6.3.1.2). Functional class names are constructed in the manner used for esters.
P-65.6.3.4.1 When ‘E’, in R-CO-O-E, is an element from the following list: B, Al, In, Ga, Tl, Si, Ge, Sn, Pb, N(cyclic), P, As, Sb, Bi, the pseudoester is named as a traditional ester, unless other names must be selected in accordance with the seniority order of classes, in decreasing order: salts > acids > anhydrides > esters. For anhydrides, see P-65.7.
Examples:
CH3-CH2-SO2-S-Ge(CH3)3
S-(trimethylgermyl) ethanesulfonothioate (PIN)
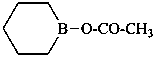
borinan-1-yl acetate (PIN)
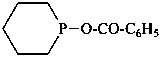
phosphinan-1-yl benzoate (PIN)
(CH3-CO-O)3B
triacetic boric trianhydride (PIN)
H2P-O-CO-CH3
acetic phosphinous anhydride (PIN)
(CH3)2N-O-CO-CH3
1-[(dimethylamino)oxy]ethan-1-one (PIN)
O-acetyl-N,N-dimethylhydroxylamine
(hydroxylamine is a preselected name; see P-68.3.1.1.1)
CH3-CO-O-P(CH3)2
acetic dimethylphosphinous anhydride (PIN; see P-67.1.3.3)
Example:
Compounds that may be considered as derived from hydroxy carboxylic acids or hydroxy sulfonic acids by loss of water intramolecularly are classified as ‘lactones’ and ‘sultones’, respectively. For these compounds heterocyclic names are preferred IUPAC names. Names derived from corresponding hydroxy acids are not recommended, but may be used in general nomenclature.
P-65.6.3.5.1 Lactones
Intramolecular esters of hydroxy carboxylic acids are ‘lactones’ and are named in three ways.
(1) as heterocyclic pseudoketones by adding the suffix ‘one’, ‘dione’, ‘thione’, etc. and the appropriate multiplicative prefixes to the name of the heterocyclic parent hydride;Method (1) gives preferred IUPAC names.(2) by changing the ‘ic acid’ ending of a systematic ‘oic acid’ name for the nonhydroxylated parent acid to ‘lactone’, and inserting a locant designating the position of the hydroxy group between the ‘o’ and ‘lactone’;
(3) by citing the term ‘carbolactone’ denoting the group –O-CO– in a ring or ring system after the name of the appropriate parent hydride preceded by a pair of locants describing the points of attachment of the carbonyl group and the oxygen atom, respectively; the locant of the carbonyl group is cited first, and, if there is a choice, is the lower locant. Multiplying prefixes and pairs of locants separated by a colon are used to indicate two or more carbolactone rings.
Examples:
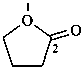
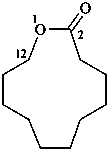
1-oxacyclododecan-2-one (PIN)
undecano-11-lactone
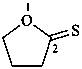
oxolane-2-thione (PIN)
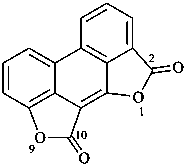
phenanthro[1,10-bc:9,8-b′c′]difuran-2,10-dione (PIN)
phenanthrene-1,10:9,8-dicarbolactone
Examples:
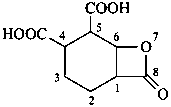
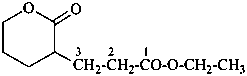
ethyl 3-(2-oxooxan-3-yl)propanoate (PIN)
ethyl 3-(2-oxo-3,4,5,6-tetrahydro-2H-pyran-3-yl)propanoate
(the saturated Hantzsch-Widman name is preferred to the hydrogenated retained name, see P-54.4.2)
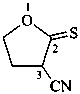
2-sulfanylideneoxolane-3-carbonitrile (PIN)
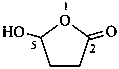
5-hydroxyoxolan-2-one (PIN)
(1) as heterocyclic heterones by adding the suffix ‘one’, ‘dione’, ‘thione’, etc. and the appropriate multiplicative prefixes to the name of the heterocyclic parent hydride;Method (1) gives preferred IUPAC names.(2) by citing the term ‘sultone’ or ‘sultine’ denoting the –O-SO2– or –O-SO– group in a ring or ring system after the name of the appropriate parent hydride preceded by a pair of locants describing the points of attachment of the sulfonyl or sulfinyl group and the oxygen atom, respectively; the locant of the sulfonyl or sulfinyl group is cited first, and, if there is a choice, is the lower locant. Multiplicative prefixes and pairs of locants separated by a colon are used to indicate two or more sultone or sultine rings.
(3) as heterocycles according to functional class names using the class name ‘oxide’
Examples:
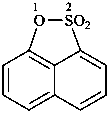
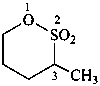
3-methyl-1,2λ6-oxathiane-2,2-dione (PIN)
3-methyl-1,2-oxathiane 2,2-dioxide
pentane-2,5-sultone
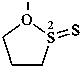
1,2λ4-oxathiolane-2-thione (PIN)
1,2-oxathiolane 2-thiooxide
Examples:
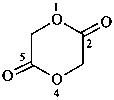
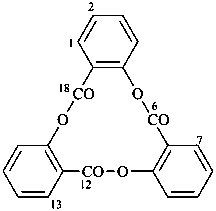
6H,12H,18H-tribenzo[b,f,j][1,5,9]trioxacyclododecine-6,12,18-trione (PIN)
(not trisalicylide)
Examples:
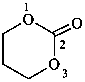
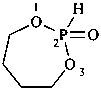
1,3,2λ5-dioxaphosphepan-2-one (PIN)
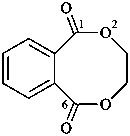
3,4-dihydro-2,5-benzodioxocine-1,6-dione (PIN)
(not 3,4-dihydrobenzo[f][1,4]dioxocine-1,6-dione)
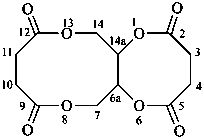
octahydro[1,4]dioxocino[2,3-c][1,6]dioxecine-2,5,9,12-tetrone (PIN)
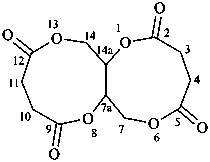
octahydro[1,5]dioxonino[3,2-b][1,5]dioxonine-2,5,9,12-tetrone (PIN)
Acylals are a class of compounds with the general structures R-CH(O-CO-R′)2, RR′C(OCOR′′)2, etc. Specific compounds are named as esters.
Example:
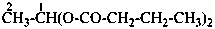
P-65.7.0 IntroductionP-65.7.0 Introduction
P-65.7.1 Symmetric anhydrides
P-65.7.2 Mixed anhydrides
P-65.7.3 Thioanhydrides and other chalcogen analogues
P-65.7.4 Peroxyanhydrides and chalcogen analogues
P-65.7.5 Diacyl derivatives of trioxidane and chalcogen analogues
P-65.7.6 Di- and polyanhydrides
P-65.7.7 Cyclic anhydrides
P-65.7.8 Polyfunctional anhydrides
Anhydrides are compounds consisting of two acyl groups bonded to the same oxygen atom, i.e., acyl-O-acyl. Symmetric and mixed anhydrides have identical and different acyl groups, respectively. The central oxygen atom can be replaced by chalcogen atoms or a peroxy group and its chalcogen analogues.
Polyanhydrides and polyfunctional anhydrides are also described in this Section.
P-65.7.1 Symmetric anhydrides
Symmetric anhydrides of monobasic acids, substituted or unsubstituted, are named by replacing the term ‘acid’ of an acid name by the class name ‘anhydride’.
Examples:
C6H5-CS-O-CS-C6H5
benzenecarbothioic anhydride (PIN)
thiobenzoic anhydride
(CH3-CH2-CH2-CH2-CH2-CO)2O
hexanoic anhydride (PIN)
CH3-CH2-CS-O-CS-CH2-CH3
propanethioic anhydride (PIN)
cyclohexanecarboxylic anhydride (PIN)
C6H5-SO2-O-SO2-C6H5
benzenesulfonic anhydride (PIN)
(Cl-CH2-CO)2O
chloroacetic anhydride (PIN)
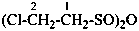
2-chloroethane-1-sulfinic anhydride (PIN)
Anhydrides derived from different monobasic acids are named by citing in alphabetical order the names of the two acids, substituted or unsubstituted, without the class name ‘acid’ followed by the class name ‘anhydride’ as a separate word.
Examples:
C6H5-SO-O-SO2-CH2-CH3
benzenesulfinic ethanesulfonic anhydride (PIN)
C6H5-CO-O-CS-CH3
benzoic ethanethioic anhydride (PIN)
benzoic thioacetic anhydride
CH3-CO-O-CO-CH2-Cl
acetic chloroacetic anhydride (PIN)
chloroacetic 4-nitrobenzene-1-sulfonic anhydride (PIN)
Examples:
C6H5-CO-O-PH2
benzoic phosphinous anhydride (PIN)
(HO)2B-O-CO-CH3
acetic boric monoanhydride (PIN)
Chalcogen analogues of anhydrides having the general structure –CO-X-CO–, –CO-X-CS–, or –CS-X-CS–, where X is –S –, –Se –, or –Te–, are named using the class names ‘thioanhydride’, ‘selenoanhydride’, or ‘telluroanhydride’, respectively.
Examples:
CH3-CH2-SO2-S-CS-C6H5
benzenecarbothioic ethanesulfonic thioanhydride (PIN)
ethanesulfonic thiobenzoic thioanhydride
4-chlorocyclohexane-1-carbothioic thioanhydride (PIN)
CH3-CO-Se-CO-CH3
acetic selenoanhydride (PIN)
Examples:
CH3-CO-O-CS-CH2-CH3
acetic propanethioic anhydride (PIN)
acetic thiopropionic anhydride
CH3-CO-S-CO-CH2-CH3
acetic propanoic thioanhydride (PIN)
acetic propionic thioanhydride
CH3-CS-O-CO-CH2-CH3
ethanethioic propanoic anhydride (PIN)
propionic thioacetic anhydride
CH3-CS-O-CS-CH2-CH3
ethanethioic propanethioic anhydride (PIN)
thioacetic thiopropionic anhydride
CH3-CS-S-CS-CH2-CH3
ethanethioic propanethioic thioanhydride (PIN)
thioacetic thiopropionic thioanhydride
CH3-CH2-CS-Se-CO-CH3
acetic propanethioic selenoanhydride (PIN)
acetic thiopropionic selenoanhydride
CH3-CS-S-CO-CH2-CH3
ethanethioic propanoic thioanhydride (PIN)
propionic thioacetic thioanhydride
Peroxyanhydrides, R-CO-OO-CO-R or R-CO-OO-COR′, are named by replacing the term ‘acid’ of an acid or two different acids by the class name ‘peroxyanhydride’.
Example:
Examples:
CH3-CO-S-O-CO-CH2-CH3
S-acetic O-propanoic thioperoxyanhydride (PIN)
CH3-CO-SS-CO-CH3
acetic dithioperoxyanhydride (PIN)
P-65.7.5.1 Anhydrides derived from peroxy acids and their chalcogen analogues are named substitutively as pseudoketones (see P-64.3). Multiplicative names are preferred when the conditions for their use are fulfilled (see P-15.3).
Examples:
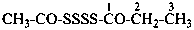
1-(acetyltetrasulfanyl)propan-1-one (PIN)
CH3-CO-S-O-S-CO-CH3
1,1′-dithioxanediyldi(ethan-1-one) (PIN)
CH3-CO-OO-S-CO-CH3
1-[(acetylperoxy)sulfanyl]ethan-1-one (PIN)
{not 1-[(acetylsulfanyl)peroxy]ethan-1-one;
the PIN has the lower alphanumerical order}
Example:
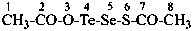
Di- and polyanhydrides have two or more –CO-O-CO– or related groups, such as –SO2-O-SO2–, respectively. They are named using the class name ‘dianhydride’, ‘trianhydride’, etc., preceded by the names of the acid groups cited as separate words.
P-65.7.6.1 Dianhydrides are named by citing the acid groups in their order of occurrence in the structure beginning with the end acid group lower in alphabetical order followed by the class term ‘dianhydride’. The numerical prefix ‘di-’ is used to generate preferred IUPAC names.
Examples:
CH3-CO-O-CO-CH2-CH2-CO-O-CO-CH3
diacetic butanedioic dianhydride (PIN)
CH3-CO-O-SO2-CH2-SO2-O-CO-CH2-CH3
acetic methanedisulfonic propanoic dianhydride (PIN)
CH3-CH2-CO-O-CO-CH2-CH2-CO-O-CO-CH2-CH2-CH3
butanoic butanedioic propanoic dianhydride (PIN)
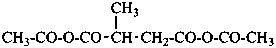
diacetic 2-methylbutanedioic dianhydride (PIN)
(1) by selecting the preferred dicarboxylic acid and citing the adjoining acid groups one of which will be substituted using the principles of substitutive nomenclature;When there is a choice the second acid group will be the acid group lower in alphabetical order.(2) by citing the acid groups, in their order of appearance, in the structure beginning with the end acid group lower in alphabetical order followed by a class term ‘dianhydride’, ‘trianhydride’, etc. Numerical prefixes are in preferred IUPAC names.
Method (1) generates preferred IUPAC names.
Examples:
CH3-CO-O-CO-CH2-CO-O-CO-CH2-CH2-CO-O-CO-CH3
(1) acetic 3-(acetyloxy)-3-oxopropanoic butanedioic dianhydride (PIN)
(2) acetic butanedioic propanedioic acetic trianhydride
Examples:
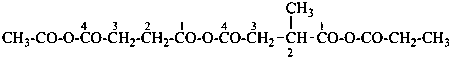
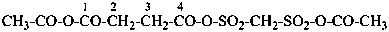
acetic butanedioic [(acetyloxy)sulfonyl]methanesulfonic dianhydride (PIN)
acetic butanedioic methanedisulfonic acetic trianhydride
Examples:
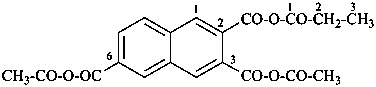
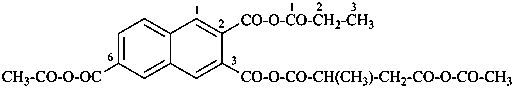
6-acetic 3-[4-(acetyloxy)-2-methyl-4-oxobutanoic] 2-propanoic naphthalene-2,3,6-tricarboxylic trianhydride (PIN)
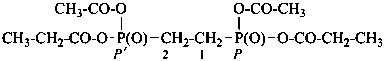
P,P′-diacetic P,P′-dipropanoic (ethane-1,2-diyl)bis(phosphonic) tetraanhydride (PIN)
3,6-diacetic 2-propanoic 8-[2-(acetyloxy)-2-oxoethyl]naphthalene-2,3,6-tricarboxylic trianhydride (PIN)
5-(acetyloxy)-5-oxopentanoic 6-(butanoyloxy)-6-oxohexanoic 4-oxo-4-(propanoyloxy)butanoic phosphoric trianhydride (PIN)
When chalcogen atoms are present in di- and polyanhydrides, their names are formed in different ways.
P-65.7.6.4.1 When all anhydride linkages are identical, as in –CO-S-CO–, names are formed using class names such as ‘thioanhydride’ preceded by the multiplicative prefixes ‘bis-’, ‘tris-’, etc.
Examples:
CH3-CO-S-SO2-CH2-SO2-S-CO-CH3
diacetic methanedisulfonic bis(thioanhydride) (PIN)
Examples:
3,6-diacetic 2-propanoic naphthalene-2,3,6-tricarboxylic tris(thioanhydride) (PIN)
acetic 3-[(acetylsulfanyl)carbonyl]naphthalene-2-carboxylic anhydride (PIN)
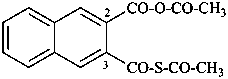
acetic 1-[3-(acetylsulfanyl)-3-oxopropyl]naphthalene-2-carboxylic anhydride (PIN)
Examples:
CH3-CO-O-CS-CH2-CH2-CS-O-CO-CH3
diacetic butanebis(thioic) dianhydride (PIN)
CH3-CO-O-CS-CH2-CH2-CS-S-CS-CH3
acetic 4-[(ethanethioyl)sulfanyl]-4-sulfanylidenebutanethioic anhydride (PIN)
CH3-CO-O-CO-CH2-CH2-CO-O-CO-CH2-CH2-CO-S-CO-CH2-CH3
acetic butanedioic 4-oxo-4-(propanoylsulfanyl)butanoic dianhydride (PIN)
P-65.7.7.1 Cyclic anhydrides formed from two acid groups attached to the same parent hydride structure are named in two ways:
(1) as heterocyclic pseudoketones;Method (1) generates preferred IUPAC names(2) by changing the class term ‘acid’ to ‘anhydride’ in the systematic or retained name of the dibasic acid.
Examples:
3-methyloxolane-2,5-dione (PIN)
3-methyl-3,4-dihydrofuran-2,5-dione
2-methylbutanedioic anhydride
methylsuccinic anhydride
furan-2,5-dione (PIN)
maleic anhydride
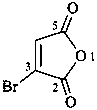
3-bromofuran-2,5-dione (PIN)
bromomaleic anhydride
2-benzofuran-1,3-dione (PIN)
isobenzofuran-1,3-dione
phthalic anhydride
5-nitro-2-benzofuran-1,3-dione (PIN)
5-nitroisobenzofuran-1,3-dione
4-nitrophthalic anhydride
1H,3H-benzo[de][2]benzopyran-1,3-dione (PIN)
1H,3H-benzo[de]isochromene-1,3-dione
naphthalene-1,8-dicarboxylic anhydride
1,8,8-trimethyl-3-oxabicyclo[3.2.1]octane-2,4-dione (PIN)
(also known as camphoric anhydride)
1,3-dioxooctahydro-2-benzofuran-4,5-dicarboxylic acid (PIN)
1,3-dioxooctahydroisobenzofuran-4,5-dicarboxylic acid
cyclohexane-1,2,3,4-tetracarboxylic acid 3,4-anhydride
(1) as heterocyclic pseudoketones;Method (1) generates preferred IUPAC names(2) by changing the class term ‘acid’ to ‘dianhydride’ in the systematic or retained name of the tetrabasic acid; the locants of the pair of acid groups are separated by a semicolon.
Examples:
hexahydrobenzo[1,2-c:3,4-c′]difuran-1,3,6,8-tetrone (PIN)
cyclohexane-1,2,3,4-tetracarboxylic 1,2:3,4-dianhydride
 |  |  |
| (I) | (II) | (III) |
| tetrahydro-4,8-ethanopyrano[4,3-c]pyran-1,3,5,7-tetrone (PIN) [numbering shown in (I)] {not 4,9-dioxatricyclo[4.4.2.02,7]dodecane-3,5,8,10-tetrone [numbering shown in (II)]} cyclohexane-1,2,3,4-tetracarboxylic 1,3:2,4-dianhydride [numbering shown in (III)] | ||
(1) as heterocyclic pseudoketones;Method (1) leads to the preferred IUPAC name.(2) by changing the class term ‘acid’ to ‘dianhydride’ or ‘thioanhydride’, ‘bis(thioanhydride)’, etc., in the systematic or retained name of the dibasic or tetrabasic acid.
Examples:
(1) hexahydro-1H-2-benzopyran-1,3(4H)-dithione (PIN)
(1) 3-sulfanylidene-2-benzothiophen-1-one (PIN)
3-sulfanylidenebenzo[c]thiophen-1-one
(1) 5,7-bis(sulfanylidene)-5,7-dihydro-1H,3H-thieno[3,4-f][2]benzofuran-1,3-dione (PIN)
5,7-dithioxo-5,7-dihydro-1H,3H-thieno[3,4-f]isobenzofuran-1,3-dione
(2) 1,3-bis(sulfanylidene)-1,3-dihydro-2-benzothiophene-5,6-dicarboxylic anhydride
1,3-dithioxo-1,3-dihydroisobenzothiophene-5,6-dicarboxylic anhydride
(1) tetrahydro-1H-cyclopenta[1,2-c:3,4-c′]dithiophene-1,3,4,6(3aH)-tetrone (PIN)
(2) cyclopentane-1,2,3,4-tetracaboxylic 1,2:3,4-bis(thioanhydride)
Return to:
Blue Book home page.