2,3-naphthalenediacetic acid (a conjunctive name)
2,2′-(naphthalene-2,3-diyl)diacetic acid (PIN;
a substitutive name; see P-15.6.1.4)
Division VIII Chemical Nomenclature and Structure Representation Division
P-50 IntroductionP-50 INTRODUCTION
P-51 Selecting the preferred type of IUPAC nomenclature
P-52 Selecting preferred IUPAC names and preselected names for parent hydrides
P-53 Selecting preferred retained names of parent hydrides
P-54 Selecting the preferred method for modifying the degree of hydrogenation
P-55 Selecting the preferred retained name for functional parent compounds
P-56 Selecting the preferred suffix for the principal characteristic groups
P-57 Selecting preferred and preselected prefixes for substituent group names
P-58 Selection of preferred IUPAC names
P-59 Name construction
Many compounds can have two or more names in accordance with several methods recommended by IUPAC for their formation, one of which is recommended herein as the preferred IUPAC name (PIN). This Chapter summarizes the selection rules that are recommended in Chapters P-1 through P-4 for generation of preferred IUPAC names for compounds described in these Chapters and also in Chapters P-6 to P-10 where applicable. Substitutive nomenclature is the principal type of nomenclature for organic compounds; however, other types are recommended because substitutive nomenclature was never recommended for naming certain classes of compounds or because they represent a simplification when the substitutive names become long and cumbersome.
In Chapter P-1, several types of nomenclature are discussed. All of them are used to generate preferred IUPAC names and names for general nomenclature. Functional class nomenclature (see P-51.2) is used to generate names of well defined classes, such as acid halides and esters. Multiplicative nomenclature (see P-51.3) is used to enable several occurrences of the same parent structure in a single molecule all to be expressed as parent structures, although this is permitted only under certain restrictive conditions. When these conditions are not fulfilled, substitutive nomenclature is recommended. Skeletal replacement (‘a’) nomenclature (see P-51.4) is used to simplify substitutive names of acyclic compounds containing heteroatoms (usually by eliminating many nesting operations); it is mandatory for naming saturated heterocyclic compounds having more than ten members and in heteropolyalicyclic nonfused bridged and spiro ring systems.
In Chapter P-2, most of the rules are unequivocal because they generate preferred IUPAC names of cyclic and acyclic compounds in themselves. When rings, ring systems, and chains are composed of entities that are themselves rings or ring systems, interconnected or not by chains, phane nomenclature is recommended to generate preferred IUPAC names; the selection of these names is discussed in P-52.2.5. The selection of preferred IUPAC names for ring assemblies is treated in P-52.2.7. The selection of preferred IUPAC names is also necessary for substituent groups derived from the parent hydrides described in Chapter P-2.
In Chapter P-3, the level of saturation expressed by the prefixes ‘hydro/dehydro’ or ‘ene/yne’ endings is considered. In the cases of characteristic groups cited as prefixes and functional parent compounds, the selection is between retained names and systematic names as components of preferred IUPAC names.
In Chapter P-4, the various seniority orders described are unequivocal, with the exception of substituted parent structures that must be analyzed as preferred IUPAC names. In P-44, comprehensive rules for the selection of a preferred parent structure are presented. A new concept for IUPAC nomenclature is described in Section P-45 called ‘Selection of preferred IUPAC names’. The selection of a preferred IUPAC name is based on hierarchical rules based on seniority orders for determining the one and only preferred parent name based on the senior parent structure described in P-44. This issue is discussed in P-58.
P-51 SELECTING THE PREFERRED IUPAC TYPE OF NOMENCLATURE
P.51.0 IntroductionP-51.0 INTRODUCTION
P-51.1 Selecting the preferred type of nomenclature
P-51.2 Functional class nomenclature (see P-15.2)
P-51.3 Multiplicative nomenclature (see P-15.3)
P-51.4 Skeletal replacement (‘a’) nomenclature (see P-15.4)
P-51.5 Conjunctive nomenclature vs. substitutive nomenclature
When a choice is needed between the several types of IUPAC nomenclature, the following selection rules must be applied. Sections P-51.1 through P-51.4 give specific rules for each type of nomenclature and examples.
P-51.1 SELECTING THE PREFERRED TYPE OF NOMENCLATURE
When there is a choice between two types of nomenclature, the preferred type of nomenclature is selected in accordance with the following rules.
P-51.1.1 Substitutive nomenclature is preferred to functional class nomenclature, except for the classes described in P-51.2 for which no substitutive names are prescribed.
Example:
Example:

Example:
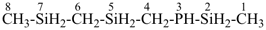
Example:
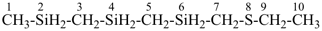
Example:
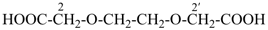
In many cases, functional class nomenclature and substitutive nomenclature can be used to give two names to one compound, for example methyl bromide, a functional class name, and bromomethane, a substitutive name, for CH3-Br. Substitutive names have now replaced many functional class names, but not all. In the context of preferred IUPAC names, it is essential to correctly use the two types of nomenclature. In P-51.2.1, the functional class names that are preferred IUPAC names are given. In P-51.2.2 the functional class names that may be used in general nomenclature are discussed and exemplified; for the substitutive names that are the preferred IUPAC names corresponding to these functional class names, see P-15.2.
P-51.2.1 Functional class nomenclature is used to generate preferred IUPAC names for the following characteristic groups.
P-51.2.2 Functional class nomenclature for general nomenclature
A certain number of classes can still be named for general nomenclature by applying functional class nomenclature. They are described in P-15.2. For these classes, preferred IUPAC names are substitutive names.
Examples:
P-51.3 MULTIPLICATIVE NOMENCLATURE (see P-15.3)
Multiplicative nomenclature is used to name assemblies of identical units linked by di- or polyvalent groups formed according to P-15.3.2. This subsection describes the formation of preferred IUPAC multiplicative names according to the principles and rules discussed in P-15.3. Substitutive nomenclature is used when the conditions for constructing multiplicative names are not met. Further, skeletal replacement (‘a’) nomenclature (see P-15.4) and phane nomenclature (see P-26) are used rather than multiplicative nomenclature when the conditions for their use are met in order to simplify name construction when multiplicative names become complex and cumbersome.
P-51.3.1 Preferred IUPAC multiplicative names
For a multiplicative name to be categorized as an IUPAC preferred name, certain restrictive conditions must be met. Multiplicative nomenclature is preferred to substitutive nomenclature for generating preferred IUPAC names to express multiple occurrences of identical parent structures, other than alkanes when
(2) the multiplicative groups, other than the central multiplicative group, are symmetrically substituted; and
(3) the locants of all substituent groups on the identical parent structures, including suffix groups, are identical.
The first two specific conditions are related to the linking di- or polyvalent groups. They are defined and exemplified in Section P-15.3.1.2. Simple and concatenated groups are used when the conditions expressed in P-15.3.1.2.1 and P-15.3.1.2.2 are fulfilled.
Examples:
P-51.3.2.1 A maximum number of identical parent structures must be expressed by the multiplicative name.
Examples:
Examples:
Example:
P-51.3.3 When conditions (1), (2), and (3) as defined in P-51.3.1, above, are not met, the preferred IUPAC name is generated by substitutive nomenclature principles.
Examples:
H3Si-SiH2-CH2-OO-SiH2-SiH3
P-51.4 SKELETAL REPLACEMENT (‘a’) NOMENCLATURE
Skeletal replacement (‘a’) nomenclature is used to generate preferred IUPAC names in place of substitutive or multiplicative names when four or more heterounits are present in an acyclic chain (see P-51.3.1). Skeletal replacement (‘a’) nomenclature is the only recommended method for certain types of cyclic compounds.
P-51.4.1 Skeletal replacement (‘a’) nomenclature in acyclic chains
P-51.4.1.1 Skeletal replacement (‘a’) nomenclature rather than substitutive or multiplicative names must be used to generate preferred IUPAC names for acyclic structures when four or more heterounits are present in a unbranched chain containing at least one carbon atom and when none of the heteroatoms constitute all or part of the principal characteristic group of the compound.
A heterounit is a set of heteroatoms having a name of its own such as, –SS–, disulfanediyl; –SiH2-O-SiH2–, disiloxane-1,3-diyl; –SOS–, dithioxanediyl (not –OSiH2O– nor –OSO– that correspond to three consecutive units ‘oxysilanediyloxy’ and ‘oxysulfanediyloxy’, respectively). Acids such as carbonic acid or phosphorus, arsenic, and antimony acids, when representing the parent compound or the principal group, are not considered as units. In presence of a characteristic group having seniority for citation as a suffix, the group –O-P(O)(OCH3)-O– is composed of three units (see example 11 below).
P-51.4.1.2 Skeletal replacement (‘a’) nomenclature generates new acyclic parent hydrides whose numbering is fixed, as it is for heterocyclic rings and ring systems. Suffixes, endings, and prefixes are added in accordance with this fixed numbering.
Examples:
(CH3)3C-OO-Si(CH3)2-O-CO-CH2-CH3
CH3-O-PH(O)-O-CH2-O-CH3
Examples:
Amine oxides (CH3)3NO
N,N-dimethylmethanamine N-oxide (PIN; P-62.5)
(trimethylazaniumyl)oxidanide
(N,N-dimethylmethanaminiumyl)oxidanide Imine oxides CH2=N(O)Cl
N-chloromethanimine N-oxide (PIN; P-62.5)
[chloro(methylidene)azaniumyl]oxidanide
(N-chloromethaniminiumyl)oxidanide Acyl halides CH3-CO-Cl
acetyl chloride (PIN; P-65.5.1) Acyl azides CH3-CH2-CH2-CO-N3
butanoyl azide (PIN; P-65.5.2.1) Acyl cyanides CH3-CH2-CO-CN
propanoyl cyanide (PIN; P-65.5.2.1) Acyl isocyanides C6H5-CO-NC
benzoyl isocyanide (PIN; P-65.5.2.1) Acyl isocyanates
(same for S, Se, Te)CH3-CO-NCO
acetyl isocyanate (PIN; P-65.5.2.1 Esters CH3-CO-O-CH3
methyl acetate (PIN; P-65.6.3.2.1) Anhydrides CH3-CO-O-CO-CH2-CH3
acetic propanoic anhydride (PIN; P-65.7.2) Acid halides, pseudohalides
[derived from class 7(c) acids]CH3-N(O)Cl2
methylazonic dichloride (PIN; P-67.1.2.5) Acid amides
[derived from class7(d) acids]CH3-NH-SO-NH2
N-methylsulfurous diamide (PIN; P-67.1.2.6) Acid hydrazides
[derived from class 7(c) acids](CH3)2P-NH-NH2
dimethylphosphinous hydrazide (PIN; P-67.1.2.6) Glycosides 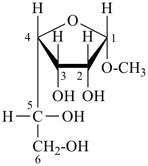
methyl α-D-gulofuranoside (P-102.5.6.2.2)
CH3-CH2-CN ethyl cyanide
propanenitrile (PIN) C6H5-NC phenyl isocyanide
isocyanobenzene (PIN) (CH3)2C=N-N=C(CH3)2 acetone azine (see P-68.3.1.2.3)
di(propan-2-ylidene)hydrazine (PIN) CH3-CH2-CH=N-OH propanal oxime
N-propylidenehydroxylamine
N-hydroxypropan-1-imine (PIN) (CH3)2C=N-NH-CO-NH2 acetone semicarbazone
2-(propan-2-ylidene)hydrazinecarboxamide (PIN)In these recommendations, identical parent structures do not have to have a principal characteristic group in order to construct a multiplicative name, which was necessary in earlier recommendations. (1) the linking bonds (single or multiple) between the central substituent group of the multiplicative group and all subsequent structural units are identical and
In these recommendations, all substituent groups, including the principal characteristic groups must be identical and have the same locant in order to construct a multiplicative name. This is a change from earlier recommendations where such locants did not need to be identical. 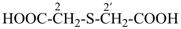
2,2′-sulfanediyldiacetic acid (PIN, multiplicative name)
[(carboxymethyl)sulfanyl]acetic acid (substitutive name)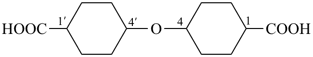
4,4′-oxydi(cyclohexane-1-carboxylic acid) (PIN, multiplicative name)
4-[(4-carboxycyclohexyl)oxy]cyclohexane-1-carboxylic acid (substitutive name)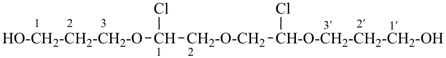
3,3′-{oxybis[(1-chloroethane-2,1-diyl)]oxy}di(propan-1-ol) (PIN,
a multiplicative name)
3-{2-[2-chloro-2-(3-hydroxypropoxy)ethoxy]-1-chloroethoxy}propan-1-ol
(a substitutive name)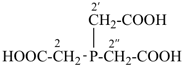
2,2′,2′′-phosphanetriyltriacetic acid (PIN, a multiplicative name)
[bis(carboxymethyl)phosphanyl]acetic acid (a substitutive name)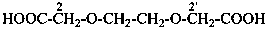
2,2′-[ethane-1,2-diylbis(oxy)]diacetic acid (PIN, a multiplicative name)
[2-(carboxymethoxy)ethoxy]acetic acid (a substitutive name)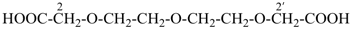
2,2′-[oxybis(ethane-2,1-diyloxy)]diacetic acid (PIN, a multiplicative name)
{2-[2-(carboxymethoxy)ethoxy]ethoxy}acetic acid (a substitutive name)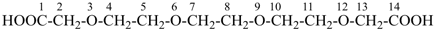
3,6,9,12-tetraoxatetradecane-1,14-dioic acid [PIN,
a skeletal replacement (‘a’) name]
2,2′-[ethane-1,2-diylbis(oxyethane-2,1-diyloxy)]diacetic acid
(a multiplicative name)
(2-{2-[2-(carboxymethoxy)ethoxy]ethoxy}ethoxy)acetic acid (a substitutive name)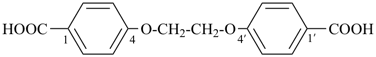
4,4′-[ethane-1,2-diylbis(oxy)]dibenzoic acid (PIN, a multiplicative name)
4-[2-(4-carboxyphenoxy)ethoxy]benzoic acid (a substitutive name)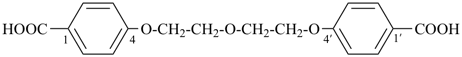
4,4′-[oxybis(ethane-2,1-diyloxy)]dibenzoic acid (PIN, a multiplicative name)
4-{2-[2-(4-carboxyphenoxy)ethoxy]ethoxy}benzoic acid (a substitutive name)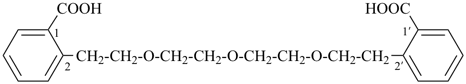
2,2′-[oxybis(ethane-2,1-diyloxyethane-2,1-diyl)]dibenzoic acid (PIN,
a multiplicative name)
2-[2-(2-{2-[2-(2-carboxyphenyl)ethoxy]ethoxy}ethoxy)ethyl]benzoic acid
(a substitutive name)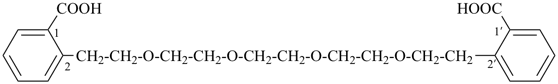
2,2′-(3,6,9,12-tetraoxatetradecane-1,14-diyl)dibenzoic acid (PIN,
a multiplicative name using the skeletal replacement (‘a’) name as the multiplying substituent group)
2,2′-[ethane-1,2-diylbis(oxyethane-2,1-diyloxyethane-2,1-diyl)]dibenzoic acid
(a multiplicative name using simple substitutive nomenclature)
2-{2-[2-(2-{2-[2-(2-carboxyphenyl)ethoxy]ethoxy}ethoxy)ethoxy]ethyl}benzoic acid (a substitutive name)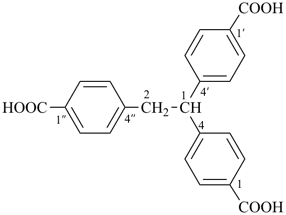
4,4′,4′′-(ethane-1,1,2-triyl)tribenzoic acid (PIN)
[not 4,4′′-[2-(4-carboxyphenyl)ethane-1,1-diyl]dibenzoic acid;
the preferred IUPAC name multiplies more identical parent structures ‘3’ vs. ‘2’; see P-15.3.3.2.1]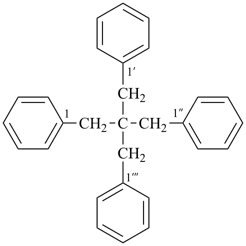
1,1′-(2,2-dibenzylpropane-1,3-diyl)dibenzene (PIN, a multiplicative name;
a multiplicative prefix name such as neopentanetetrayl has never been recognized)
(2,2-dibenzyl-3-phenylpropyl)benzene (substitutive name)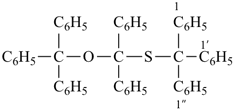
1,1′,1′′-({[diphenyl(triphenylmethoxy)methyl]sulfanyl}methanetriyl)tribenzene (PIN)
[not 1,1′-{(triphenylmethoxy)[(triphenylmethyl)sulfanyl]methylene}dibenzene];
this name multiplies only two identical parent structures]
[not 1,1′,1′′-({diphenyl[(triphenylmethyl)sulfanyl]methoxy}methanetriyl)tribenzene;
the
PIN is lower alphabetically
(‘diphenyltriphenylmethoxy’ is lower alphabetically than ‘diphenyltriphenylmethyl’)]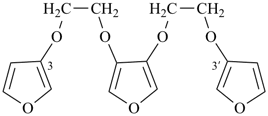
3,3′-[furan-3,4-diylbis(oxyethane-2,1-diyloxy)]difuran (PIN, a multiplicative name)
3,4-bis[2-(furan-3-yloxy)ethoxy]furan (a substitutive name)]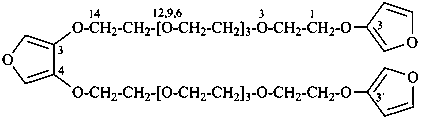
3,3′-{furan-3,4-diylbis[oxy(3,6,9,12-tetraoxatetradecane-14,1-diyl)oxy]}difuran (PIN)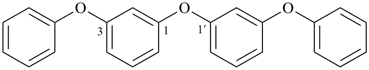
1,1′-[oxybis(3,1-phenyleneoxy)dibenzene (a multiplicative name)
2,4,6-trioxa-1,7(1),3,5(1,3)-tetrabenzenaheptaphane (PIN, a phane name, see P-51.4, P-52.2.5)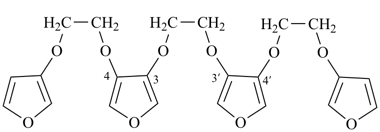
3,3′-[ethane-1,2-diylbis(oxy)]bis{4-[2-(furan-3-yloxy)ethoxy]furan} (a multiplicative name)
[not 3,3′-[ethane-1,2-diylbis(oxyfuran-4,3-diyloxyethane-2,1-diyloxy)]difuran (a multiplicative name)]
2,5,7,10,12,15-hexaoxa-1,16(3),6,11(3,4)-tetrafuranahexadecaphane (PIN; a phane name, see P-51.4, P-52.2.5)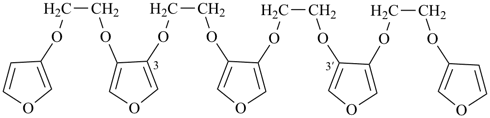
3,3′-[furan-3,4-diylbis(oxyethane-2,1-diyloxy)]bis{4-[2-(furan-3-yloxy)ethoxy]furan}
(a multiplicative name)
[not 3,4-bis[2-({4-[2-(furan-3-yloxy)ethoxy]furan-3-yl}oxy)ethoxy]furan (a substitutive name)]
[not 3,3′-[furan-3,4-diylbis(oxyethane-2,1-diyloxyfuran-4,3-diyloxyethane-2,1-diyloxy)]difuran (a multiplicative name)]
2,5,7,10,12,15,17,20-octaoxa-1,21(3), 6,11,16(3,4)-pentafuranahenicosaphane (PIN,
a phane name, see P-51.4, P-52.2.5)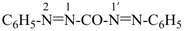
bis(phenyldiazenyl)methanone (PIN)
[not 1,1′-carbonylbis(2-phenyldiazene);
not 1,1′-[carbonylbis(diazenediyl)]dibenzene;
methanone is senior to both ‘diazene’ and a carbocyclic ring, see P-41]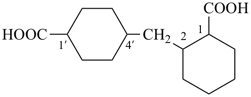
2,4′-methylenedi(cyclohexane-1-carboxylic acid) (a multiplicative name)
2-[(4-carboxycyclohexyl)methyl]cyclohexane-1-carboxylic acid (PIN,
a
substitutive name, see P-45.2.2)
[not 4-[(2-carboxycyclohexyl)methyl]cyclohexane-1-carboxylic acid;
the substituent locant ‘2’ is lower than ‘4’ (see P-14.3.5, P-45.2.2)]
[(disilanylmethyl)peroxy]disilane (PIN, a substitutive name)
[not [(disilanylperoxy)methyl]disilane;
‘disilanylmethylperoxy’ precedes ‘disilanylperoxymethyl’ in alphanumerical order (see P-45.5)]In these recommendations, groups of atoms having a simple multivalent name are considered as a unit, hence the term heterounits includes both heteroatoms and heterogroups. Heterogroups were not considered as a single heterounit in previous recommendations. Fixed numbering for heteroacyclic parent structures named by skeletal replacement (‘a’) nomenclature is a major change to Rule C-0.6 (ref. 1) where principal characteristic groups and free valence were preferred over heteroatoms for low locants. 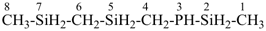
3-phospha-2,5,7-trisilaoctane [PIN, skeletal replacement (‘a’) name]
(methylsilyl)({[(methylsilyl)methyl]silyl}methyl)phosphane (a substitutive name)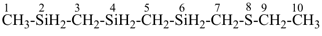
8-thia-2,4,6-trisiladecane [PIN, skeletal replacement (‘a’) name]
1-[(ethylsulfanyl)methyl]-1′-methyl-1,1′-[silanediylbis(methylene)]bis(silane); (a multiplicative name)
({[(ethylsulfanyl)methyl]silyl}methyl)[(methylsilyl)methyl]silane; (a substitutive name)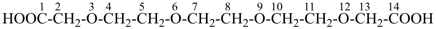
3,6,9,12-tetraoxatetradecanedioic acid [PIN, a skeletal replacement (‘a’) name]
2,2′-{ethane-1,2-diylbis[(oxyethane-2,1-diyl)oxy]diacetic acid; (a multiplicative name)
2-(2-{2-[2-(carboxymethoxy)ethoxy]ethoxy}ethoxy)acetic acid; (a substitutive name)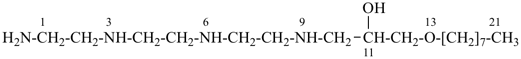
1-amino-13-oxa-3,6,9-triazahenicosan-11-ol [PIN; a skeletal replacement (‘a’) name]
1-{[2-({2-[(2-aminoethyl)amino]ethyl}amino)ethyl]amino}-3-(octyloxy)propan-2-ol; (a substitutive name)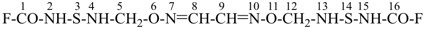
6,11-dioxa-3,14-dithia-2,4,7,10,13,15-hexaazahexadeca-7,9-dienedioyl difluoride (PIN)
(a skeletal replacement name)
(an acyclic dioyl fluoride is preferred to a multiplied carbamoyl fluoride)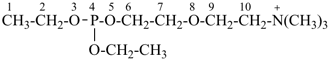
4-ethoxy-N,N,N-trimethyl-3,5,8-trioxa-4-phosphadecan-10-aminium (PIN) 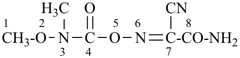
7-cyano-3-methyl-4-oxo-2,5-dioxa-3,6-diazaoct-6-en-8-amide (PIN)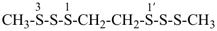
1,1′-(ethane-1,2-diyl)bis(3-methyltrisulfane) (PIN)
(not 2,3,4,7,8,9-hexathiadecane; trisulfane, HS-S-SH, is
a parent hydride and is not allowed to be a heterounit)
(tert-butylperoxy)dimethylsilyl propanoate (PIN)
(not 2,2,5,5-tetramethyl-3,4,6-trioxa-5-silanonan-7-one;
only two heterounits are present: –OO– and –Si–;
the principal characteristic group is an ester and the –O– is a part of it)
methoxymethyl methyl phosphonate (PIN)
[not 2,4,6-trioxa-3λ5-phosphaheptan-3-one;
the three heteroatoms –O-P-O– are part of an ester and are expressed
as the principal characteristic group; this leaves only one heterounit, –O–,
and skeletal replacement (‘a’) nomenclature cannot be used as the PIN]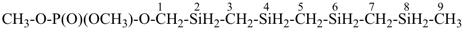
dimethyl 2,4,6,8-tetrasilanonan-1-yl phosphate (PIN)
[three of the heteroatoms –O-P-O– are part of the ester expressed as the principal characteristic group;
however there are four silicon atoms in one of the organyl parts of the ester
and skeletal replacement (‘a’) nomenclature is used to name this ester group]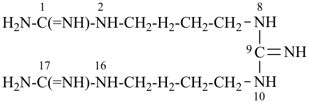
9-imino-2,8,10,16-tetraazaheptadecanediimidamide (PIN)
(a diimidamide expressed as a principal characteristic group
is senior to a carbonimidic diamide)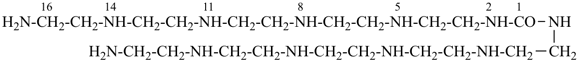
16-amino-N-(14-amino-3,6,9,12-tetraazatetradecan-1-yl)-2,5,8,11,14-pentaazahexadecanamide [PIN;
an amide expressed as a principal characteristic group is senior to
urea, a carbonic diamide, or an amine expressed as a principal characteristic group;
since four heteroatoms are also present in the N-substituent group, it must also be named by skeletal replacement (‘a’) nomenclature]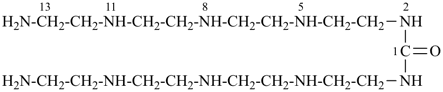
13-amino-N-(2-{[2-({2-[(2-aminoethyl)amino]ethyl}amino)ethyl]amino}ethyl)-2,5,8,11-tetraazatridecanamide (PIN;
an amide expressed as
characteristic group is senior to urea, a carbonic diamide, and to an amine expressed as a characteristic group;
since only three heteroatoms are present in the N-substituent group, it must be named substitutively)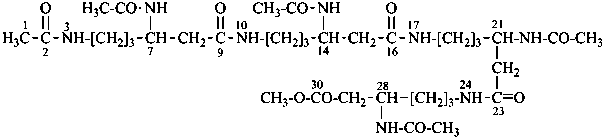
methyl 7,14,21,28-tetraacetamido-2,9,16,23-tetraoxo-3,10,17,24-tetraazatriacontan-30-oate (PIN;
an ester is senior to an amide or a ketone)
In these recommendations heterochains may be terminated by certain heteroatoms and not just by carbon atoms. Previous recommendations required a heterochain to be terminated by carbon atoms.
Example:
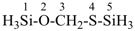
Skeletal replacement (‘a’) nomenclature is the only recommended method to generate names for certain heterocyclic compounds.
P-51.4.2.1 Skeletal replacement (‘a’) nomenclature is used to derive preferred IUPAC names for heteromonocyclic compounds having more than ten ring atoms (see P-22.2.3).
Skeletal replacement (‘a’) nomenclature is also possible with rings having less than 10 ring members if the skeletal replacement (‘a’) prefix represents a metal as defined in P-69.4.
Adapting the principles of the Hantzsch-Widman system to the elements of Groups 1-12 in and including their skeletal replacement (‘a’) prefixes would be a major change from previous recommendations even though the names of such organometallic compounds involving these elements are not PINs at this time.
Examples:
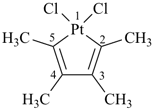
1,1-dichloro-2,3,4,5-tetramethylplatinole (Hantzsch-Widman name)
1,1-dichloro-2,3,4,5-tetramethyl-1-platinacyclopenta-2,4-diene
(a skeletal replacement name; see P-69.4)
The option to include the metallic elements in addition to the metals in Groups 13 through 16 in the Hantzsch-Widman system and their skeletal replacement (‘a’) prefixes (see P-69.4) is a major change from previous recommendations for the Hantzsch-Widman system.
P-51.4.2.2 Skeletal replacement (‘a’) nomenclature is used to derive preferred IUPAC names for heterocyclic von Baeyer ring systems (see P-23.3.1).
Example:
Example:
Example:
P-51.4.2.6 When necessary, the choice of a preferred IUPAC name of a heterocyclic parent structure is made before the insertion of skeletal replacement (‘a’) prefixes. This is the case with assemblies of identical heterocyclic compounds (see P-28.4) composed of heterocyclic compounds of the von Baeyer type and with monocyclic compounds having more than ten members.
Examples:
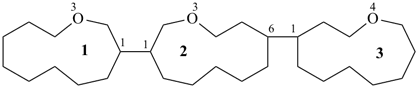
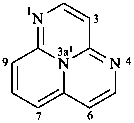
23-thia-12,22:26,32-terbicyclo[2.2.1]heptane (PIN; see P-28.3.1)
{not 2,6-bis(bicyclo[2.2.1]heptan-2-yl)-3-thiabicyclo[2.2.1]heptane}
3′-thia-2,2′:6′,2′′-terbicyclo[2.2.1]heptane
Example:
When there is a choice between conjunctive nomenclature and substitutive nomenclature, preferred IUPAC names are formed by using substitutive nomenclature (including multiplicative nomenclature and skeletal replacement nomenclature when the conditions for their use are fulfilled) (see P-51).
Examples:
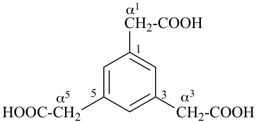
benzene-1,3,5-triacetic acid
2,2′,2′′-(benzene-1,3,5-triyl)triacetic acid (PIN)
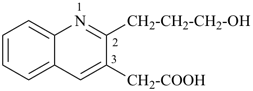
2-(3-hydroxypropyl)quinoline-3-acetic acid
[2-(3-hydroxypropyl)quinolin-3-yl]acetic acid (PIN)
(a carboxylic acid is senior to an alcohol)
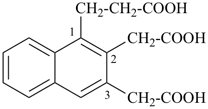
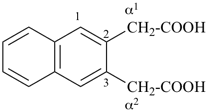
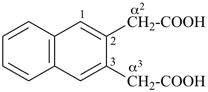
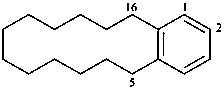
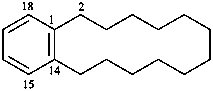
1-(2-carboxyethyl)naphthalene-2,3-diacetic acid
3-[2,3-bis(carboxymethyl)naphthalen-1-yl]propanoic acid (PIN)
For naming the parent hydrides described in Chapter P-2, when only one method is described, the resulting single names are naturally preferred IUPAC names. When more than one method is recommended for generating the names of parent hydrides, preferred IUPAC names, and in some cases preselected names, must be chosen. Some retained names are used as preferred IUPAC names and as names for use in general nomenclature.
P-52.1 Selecting preselected names
P-52.2 Selecting preferred IUPAC names
P-52.1 SELECTING PRESELECTED NAMES
P-52.1.1 Mononuclear parent hydride names are listed in P-21.1.1. Phosphane, PH3, arsane, AsH3, stibane, SbH3, and bismuthane, BiH3, are preselected names; the names phosphine, arsine, stibine, and bismuthine, respectively, are retained for use in general nomenclature.
P-52.1.2 Preselected names for homogeneous acyclic polynuclear parent hydrides are described in P-21.2.2. The preselected name for NH2-NH2 is the retained name hydrazine; the systematic name diazane may be used in general nomenclature.
P-52.1.3 Preselected names for heterogeneous acyclic parent hydrides composed of alternating ‘ab’ atoms, i.e., [a(ba)n parent hydrides], except carbon or halogen, are described in P-21.2.3.1.
In these recommendations, the ‘amine’ characteristic group is recognized in a(ba)n parent hydrides, which is a change from earlier recommendations where it was not recognized. In addition, carbon was not excluded as a ‘b’ element leading to conflicts with the order of priority for heteranes.
Examples:
CH3-NH-CH3
N-methylmethanamine (PIN)
(not dicarbazane)
HSe-S-Se-S-SeH
triselenathiane (preselected name)
SiH3-NH-SiH2-NH-SiH3
N,N′-disilylsilanediamine (based on silane, a preselected name)
(not trisilazane)
Examples:
AsH5
λ5-arsane (preselected name)
arsorane
SH4
λ4-sulfane (preselected name)
(not sulfurane)
SH6
λ6-sulfane (preselected name)
(not persulfurane)
IH3
λ3-iodane (preselected name)
(not iodinane)
IH5
λ5-iodane (preselected name
(not periodinane)
SbH5
λ5-stibane (preselected name)
stiborane
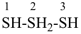
2λ4-trisulfane (preselected name)
The final ‘e’ in Hantzsch-Widman names is required in preferred IUPAC names; it is still optional in general nomenclature. In the 1979 Rules (ref. 1), the final ‘e’ of a Hantzsch-Widman name was omitted when there was no nitrogen in the ring; in the 1993 Guide (ref. 2) this omission was made optional.
P-52.1.5.1 Preselected names for homogeneous heteromonocyclic parent hydrides consisting of ten or fewer ring members are Hantzsch-Widman names (see P-22.2.2). Skeletal replacement (‘a’) names are preselected names for homogeneous heteromonocyclic parent hydrides with more than ten ring members (see P-22.2.3). Alternative names, which may be used in general nomenclature, are those formed by using the prefix ‘cyclo’ (see P-22.2.5).
Examples:
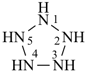
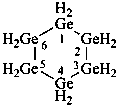
hexagerminane (preselected name)
cyclohexagermane
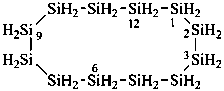
dodecasilacyclododecane (preselected name; see P-12.2)
cyclododecasilane
Preselected names for heterogeneous heteromonocyclic parent hydrides with ten or fewer ring members and consisting of alternating heteroatoms, i.e., [ab]n, are Hantzsch-Widman names (see P-22.2.2). Skeletal replacement (‘a’) names (see P-22.2.3) are preselected names for heterogeneous monoheterocyclic parent hydrides with alternating heteroatoms with more than ten ring members. Alternative names for use only in general nomenclature are those formed by using the prefix ‘cyclo’ (see P-52.1).
Examples:
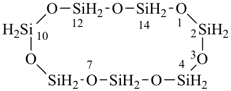
1,3,5,7,9,11,13-heptaoxa-2,4,6,8,10,12,14-heptasilacyclotetradecane (preselected name)
cycloheptasiloxane
P-52.1.6.1 Preselected names for von Baeyer compounds and for spiro compounds having only monocyclic components and consisting entirely of heteroatoms of the same kind are names formed by citing the appropriate prefix, such as ‘bicyclo’, ‘spiro’, etc., and descriptor enclosed within square brackets followed by a numerical prefix for the total number of heteroatoms and the name of the mononuclear parent hydride; alternative names for use in general nomenclature are formed by skeletal replacement (‘a’) nomenclature (see P-23.4 and P-24.2.4.2).
Examples:
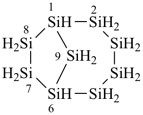
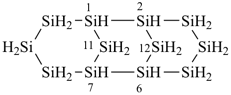
tricyclo[5.3.1.12,6]dodecasilane (preselected name)
dodecasilatricyclo[5.3.1.12,6]dodecane
Examples:
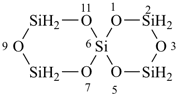
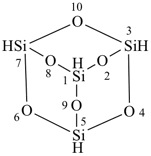
tricyclo[3.3.1.13,7]tetrasiloxane (preselected name)
2,4,6,8,9,10-hexaoxa-1,3,5,7-tetrasilaadamantane
2,4,6,8,9,10-hexaoxa-1,3,5,7-tetrasilatricyclo[3.3.1.13,7]decane
Examples:
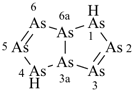
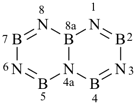
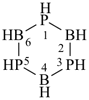
[1,3,5,2,4,6]triazatriborinino[1,2-a][1,3,5,2,4,6]triazatriborinine (preselected name)
1,3,4a,6,8-pentaaza-2,4,5,7,8a-pentaboranaphthalene (numbering shown)
P-52.2.1 Acyclic and monocyclic hydrocarbons
P-52.2.2 Heteroacyclic and heteromonocycles
P-52.2.3 Unsaturated heteromonocyclic compounds with more than ten ring members.
P-52.2.4 Preferred IUPAC names in fusion nomenclature
P-52.2.5 Preferred IUPAC names in phane nomenclature
P-52.2.6 Selecting preferred IUPAC names for (C60-Ih)[5,6]fullerene and (C70-D5h(6))[5,6]fullerene modified by ‘nor’ or ‘seco’ prefixes
P-52.2.7 Preferred IUPAC names and numbering for ring assemblies
P-52.2.8 Selection between a ring and a chain as parent hydride
P-52.2.1 Acyclic and monocyclic hydrocarbons
P-52.2.1.1 The names methane, ethane, propane, and butane are used as preferred IUPAC names for CH4, CH3-CH3, CH3-CH2-CH3, and CH3-CH2-CH2-CH3, respectively. Acetylene is the preferred IUPAC name for HC≡CH but substitution is not allowed. Limited substitution is allowed in general nomenclature, see P-15.1.8.2.2.
P-52.2.1.2 The name [n]annulene is used in preferred IUPAC names as a parent component in fusion nomenclature (see P-25.3.2.1.1) and may be used in general nomenclature as the name for the monocycle itself. Preferred IUPAC names for cycloalkenes and cycloalkapolyenes are generated from the corresponding cycloalkane names (see P-31.1.3.1).
Examples:
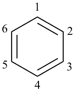
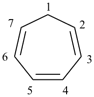
cyclohepta-1,3,5-triene (PIN)
1H-[7]annulene (preferred IUPAC name
for the parent component in fusion nomenclature, see P-25.3.2.1.1)
P-52.2.2.1 Formazan is the preferred IUPAC name retained for HN=N-CH=N-NH2. Hydrazine is the preferred name for H2N-NH2.
P-52.2.2.2 Preferred IUPAC names for heteromonocyclic rings with no more than ten ring members are Hantzsch-Widman names, including the locants ‘1,2’ and ‘1,3’. The retained names ‘oxazole’, ‘isoxazole’, ‘thiazole’, and ‘isothiazole’ are allowed in general nomenclature.
Although the final ‘e’ in Hantzsch-Widman names is required in preferred IUPAC names; it is still optional in general nomenclature. In the 1979 Rules (ref. 1), the final ‘e’ of a Hantzsch-Widman name was omitted when there was no nitrogen in the ring; in the 1993 Guide (ref. 2) this omission was made optional.
Examples:
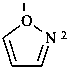
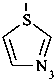
1,3-thiazole (PIN)
thiazole
Preferred IUPAC names for unsaturated monocyclic compounds with more than ten ring members derived from cycloalkanes and modified by skeletal replacement (‘a’) nomenclature are formed by changing the ‘ane’ ending of the saturated heteromonocycle to ‘ene’, ‘adiene’, etc. (see P-31.1.1). [n]Annulene names may be used in general nomenclature (see P-31.1.3.2) for the heteromonocycle itself; however, the name ‘annulene’ cannot be used to designate these heterocyclic compounds as components in fusion nomenclature.
Example:
P-52.2.4.1 Five-membered ring requirement
Fusion nomenclature gives preferred IUPAC names only to compounds having at least two rings of at least five or more members. This requirement is not necessarily applied in general nomenclature, in which names such as cyclopropabenzene and cyclobutabenzene can be used. When fusion names are not allowed, unsaturated von Baeyer ring system names are preferred IUPAC names (see P-31.1.4.2).
This is a change from the recommendation in the 1998 publication on fused ring nomenclature (see FR-0, ref. 4) and the 1993 Guide (ref. 2) where no restriction was placed on the size of the two rings that could be used in a fused ring system. For preferred IUPAC names a fusion name can only be used when at least two rings of five or more members are present; this is consistent with recommendations in the 1979 edition (ref. 1). In general nomenclature there is no restriction on the size of rings in a fused ring system.
Examples:
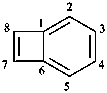
cyclobutabenzene
bicyclo[4.2.0]octa-1,3,5,7-tetraene (PIN)
Heteromonocycles having more than ten ring members and the maximum number of noncumulative double bonds whose names are denoted by the ‘ine’ ending described in P-22.2.4 are used as parent components as well as attached components in preferred IUPAC fusion names. ‘Annulene’ names modified by skeletal replacement (‘a’) nomenclature (see P-52.2.3) are not recommended for generating names of heterocyclic fusion compounds.
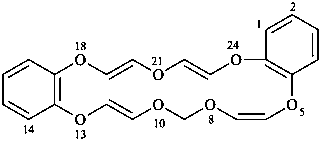
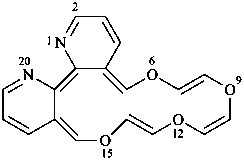
[1,4,7,10]tetraoxacyclohexadecino[13,12-b:14,15-b′]dipyridine (PIN; see P-25.3.7.1)
When two (or more) possible parent components are separated by an odd number of interparent components and these are ordered symmetrically with respect to their component rings (but not necessarily with their fusion locants), the whole system is treated as a multiparent system. In P-25.3.7.3, second- and higher-order interparent components are named using the multiplying prefixes ‘di’, ‘tri’, etc., or ‘bis’, ‘tris’, etc. Appropriate locants are assigned to interparent components, unprimed and primed for first-order interparent components, double primed for second-order interparent components, triple primed for third-order interparent components, and so on.
Example:
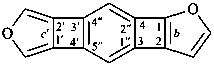
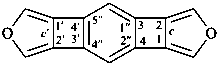
Example:
The fusion principles described in P-25.1 through P-25.3 apply to pairs of components. It is not possible by these principles to name a system in which a third component is ortho- and peri-fused to two components that are themselves ortho- or ortho- and peri-fused together. Hence, when a third component is ortho- and peri-fused to two components that are themselves ortho- or ortho- and peri-fused together, the following procedures are applied to generate a preferred IUPAC name.
P-52.2.4.4.1 Selection of a less senior parent ring or ring system
P-52.2.4.4.1.1 A less preferred parent ring or ring system component is selected that will permit a fusion name. Second and third choice of parent rings or ring system parent components may be chosen according to the seniority order for selecting the senior ring or ring system as the parent for naming the fused ring system (see P-25.3.2.4).
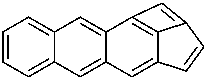
Examples:
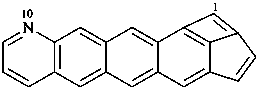
P-52.2.4.4.1.2 It should be noted that benzo heterocycles are considered as one component, thus permitting the construction of fusion names for ring systems that could not otherwise be named by fusion principles.
Example:
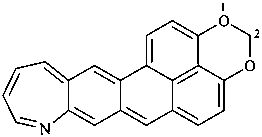
P-52.2.4.4.2 Skeletal replacement (‘a’) nomenclature
When the fusion principles discussed in P-25.1 through P-25.3 apply, no skeletal replacement (‘a’) name is recommended. This procedure is valid only for cases described here in P-52.2.4.4.2.1.
P-52.2.4.4.2.1 If the corresponding hydrocarbon fused ring system can be named by fusion principles or has a retained name, then heteroatoms are identified by skeletal replacement (‘a’) nomenclature using the appropriate ‘a’ prefixes (see P-22.2.3). The numbering of the fused hydrocarbon system is not altered by the ‘a’ prefixes.
Examples:
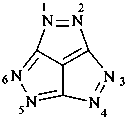
1,3a1,4,9-tetraazaphenalene (PIN)
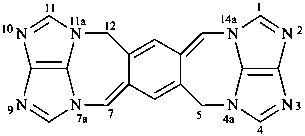
5H,12H-2,3,4a,7a,9,10,11a,14a-octaazadicyclopenta[ij:i′j′]benzo[1,2-f:4,5-f′]diazulene (PIN)
Examples:
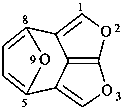
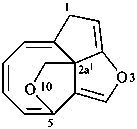
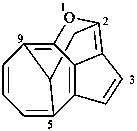
1H-3,10-dioxa-2a1,5-ethanocycloocta[cd]pentalene (PIN)
4H-9,2a1-(epoxymethano)-2-oxacycloocta[cd]pentalene
1-oxa-5,9,2-(epiethane[1,1,2]triyl)cycloocta[cd]pentalene (PIN)
5,9,2-(epiethane[1,1,2]triyl)-1-oxacycloocta[cd]pentalene
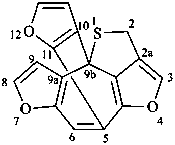
2H-4,7,12-trioxa-1-thia-5,9b-[1,2]epicyclopentadicyclopenta[cd,h]azulene (PIN)
2H-5,9b-[2,3]furano-4,7-dioxa-1-thiadicyclopenta[cd,h]azulene
Examples:
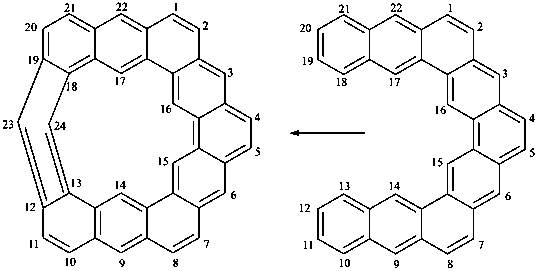
8,7-(azenoetheno)cyclohepta[4,5]cycloocta[1,2-b]pyridine (PIN)
(not 6,7-buta[1,3]dienocycloocta[1,2-b:5,6-c′]dipyridine;
the fused ring portion has the maximum number of atoms)
P-52.2.5.1 Cyclic and linear phane structures are described in section P-26. For the purpose of selecting preferred IUPAC names cyclic and acyclic phane systems are defined as follows:
(1) cyclophanes are cyclic phane structures with at least six nodes including two or more rings or ring systems not ortho or ortho and peri-fused to the cyclophane ring, and with at least one ring or ring system of which must be a mancude system;P-52.2.5.2 When the conditions given in P-52.2.5.1 for cyclic phane systems are not fulfilled, names of fused ring systems, bridged fused systems, or von Baeyer systems are preferred IUPAC names. The following subsections illustrate these situations.(2) linear phanes consist of four or more rings or ring systems, two of which must be terminal, and together with acyclic atoms or chains must consist of at least seven nodes (components).
P-52.2.5.2.1 Mancude systems attached to adjacent atoms of an alicyclic large ring.
Mancude systems attached to adjacent atoms of an alicyclic ring are either fused systems or bridged fused systems. Fusion names described in P-25.0 through P-25.3 or bridged fused ring systems described in P-25.4 are preferred IUPAC names.
Example:
The seniority order described in P-44.2.2.2 for relevant polycyclic systems is as follows: cyclic phane systems > fused ring systems > bridged fused systems > non-fused bridged systems. The following examples illustrate the application of this seniority order in the derivation of a preferred IUPAC name.
Examples:
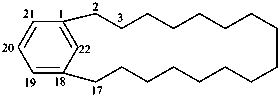
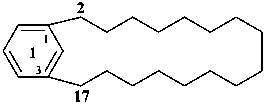
1(1,3)-benzenacycloheptadecaphane
(a phane name)
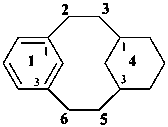
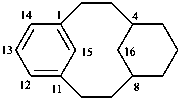
tricyclo[9.3.1.14,8]hexadeca-1(15),11,13-triene
(a von Baeyer name)
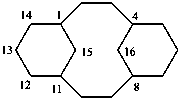
1,4(1,3)-dicyclohexanacyclohexaphane
(phane name)
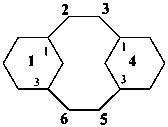
tricyclo[12.3.1.05,10]octadecane
(a von Baeyer name)
bicyclo[12.4.0]octadeca-1(14),15,17-triene
(von Baeyer name)
 |  | |
| (I) | (II) | |
| (I) 3,7-dithia-1(1,7),5(7,1)-dinaphthalenacyclooctaphane (PIN; a phane name) (II) 5,7,14,16-tetrahydro-1,17:8,10-diethenodibenzo[c,j][1,8]dithiacyclotetradecine (a bridged fused ring name) | ||
P-52.2.5.3 Ring assemblies, linear phane names, and other linear acyclic/cyclic compounds
Phane nomenclature is used to generate preferred IUPAC names for ring assemblies and linear acyclic/cyclic compounds that include a minimum of seven nodes including at least four rings or ring systems, two of which must be terminal, even though the compounds could also be named by substitutive or multiplicative nomenclature.
A new numbering system is now recommended for preferred IUPAC names of ring assemblies with more than two rings or ring systems; it consists of composite locants, for example, 12. The previous used locant system for ring assemblies with more than two rings or ring systems using serially primed locants (refs. 1 and 2) may be used in general nomenclature.
Example 1:
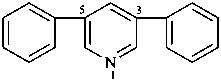
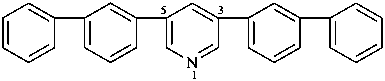
3,5-di([1,1′-biphenyl]-3-yl)pyridine (PIN, substitutive name)
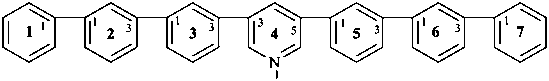
4(3,5)-pyridina-1,7(1),2,3,5,6(1,3)-hexabenzenaheptaphane (PIN, a phane name)
3,5-di([11,21:23,31-terphenyl]-13-yl)pyridine (a substitutive name, see P-28.3.1)
3,5-di([1,1′:3′,1′′-terphenyl]-3-yl)pyridine (a substitutive name)
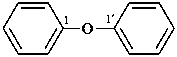
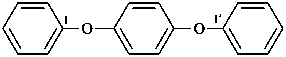
1,1′-[1,4-phenylenebis(oxy)]dibenzene (PIN, a multiplicative name)
1,4-diphenoxybenzene (a substitutive name)
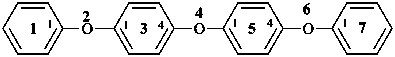
2,4,6-trioxa-1,7(1),3,5(1,4)-tetrabenzenaheptaphane (PIN, a phane name)
1,1′-oxybis(4-phenoxybenzene) (a multiplicative name)
1-phenoxy-4-(4-phenoxyphenoxy)benzene (a substitutive name)
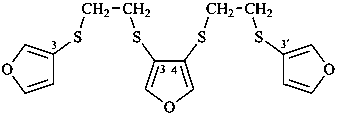
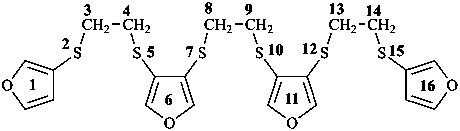
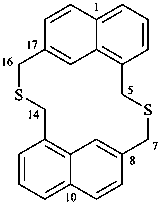
2,5,7,10,12,15-hexathia-1,16(3),6,11(3,4)-tetrafuranahexadecaphane (PIN, a phane name)
3,3′-[ethane-1,2-diylbis(sulfanediyl)]bis(4-{[2-(furan-3-ylsulfanyl)ethyl]sulfanyl}furan) (a multiplicative name)
3-{[2-(furan-3-ylsulfanyl)ethyl]sulfanyl}-4-({2-[(4-{[2-(furan-3-ylsulfanyl)ethyl]sulfanyl}furan-3-yl)sulfany]ethyl}sulfanyl)furan (a substitutive name)
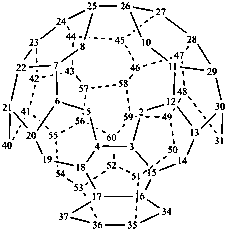
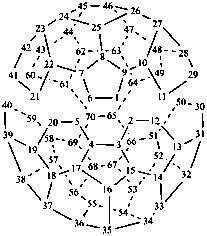
P-52.2.6.1 Systematic fused and bridged fused ring system names for structures derived from unmodified fullerenes by removal of carbon atoms and rings using a ‘nor operation’, or by removing rings by cutting bonds using a ‘seco’ operation, are often difficult to generate and even more difficult to decipher. Hence, an important objective in naming fullerene fragments is to retain as much as possible of the unmodified fullerene structure on which to base the name. To achieve this, it is recommended that, in order to receive a modified fullerene name, a fullerene fragment must be large enough so as to, arbitrarily, contain more than one-half the number of carbon atoms and more than one-third of the number of rings present in the unmodified fullerene. When these two conditions are fulfilled, a preferred IUPAC name is a modified fullerene name. If at least one of these conditions is not fulfilled the preferred IUPAC name is a fused ring or a bridged fused ring name.
Fragments of a (C60-Ih)[5,6]fullerene or a (C70-D5h(6))[5,6]fullerene derived by removal of carbon atoms or cleavage of bonds are named as norfullerenes, secofullerenes, or seconorfullerenes when both of the following two conditions are fulfilled:
(1) the fullerene fragment contains more than one-half of the carbon atoms that were present in the unmodified fullerene, i.e., at least 31 and 36 carbon atoms, respectively, for the (C60-Ih)[5,6]fullerene and (C70-D5h(6))[5,6]fullerene;P-52.2.6.2 Nor(C60-Ih)[5,6]fullerenes and nor(C70-D5h(6))[5,6]fullerenes.(2) the fullerene fragment must consist of at least one-third of the five- and/or six-membered rings that were present in the unmodified fullerene; i.e., 11 and 13 rings, respectively, for the (C60-Ih)[5,6]fullerene and (C70-D5h(6))[5,6]fullerene.
Example 1: C30H10
 |  | |
| (I) | (II) | |
| cyclopenta[cd]di-as-indaceno[3,4,5,6-fghij:3′,4′,5′,6′-lmnoa]fluoranthene (I) (PIN) [not 1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,25,26,29,30,33,34,37,38-triacontanor(C60-Ih)[5,6]fullerene (II)] | ||
Explanation: The preferred IUPAC name for this fullerene fragment is a systematic fusion ring name because it contains only 30 carbon atoms.
Example 2: C34H10
 |  | |
| (I) | (II) | |
| 1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,29,30,37,38-hexacosanor(C60-Ih)[5,6]fullerene (I) (PIN) [not bis(benzo[1,8]-as-indaceno[3,4,5,6-fghij:3′,4′,5′,6′-lmnoa])cyclopenta[cd]fluoranthene (II)] | ||
Explanation: The preferred IUPAC name for this fullerene fragment is a norfullerene name because it contains 34 carbon atoms and 13 rings.
Example 3: C36H22
 |  | |
| (I) | (II) | |
| 3,10-[2,7]epiphenanthropicene (I) (PIN) [not 7,8,9,10,16,22,23,24,25,26,27,28,32,33,34,35,36,37,38,42,43,44,45,46,47,48,52,53,54,55,56,57,67,68-tetratriacontanor(C70-D5h(6))[5,6]fullerene (II)] | ||
Explanation: The preferred IUPAC name for this fullerene fragment is a systematic bridged fused ring name because it contains only eight rings.
Example 4: C45H15
 |  | |
| (I) | (II) | |
| 7,8,9,10,22,23,24,25,26,27,28,34,35,36,42,43,44,45,46,47,48,54,55,62,63-pentacosanor(C70-D5h(6))[5,6]fullerene (I) (PIN) [not 8,7,2-(epiethane[1,2]diyl[1]ylidene)-1,9,18-(epiprop[1]ene[1,1]diyl[3]ylidene)acephenanthryleno[4,3-bc]tricyclopenta[n,pqr,tuv]picene (II)] | ||
Explanation: The preferred IUPAC name for this fullerene fragment is a norfullerene name because it has 45 carbon atoms and it has 15 rings.
Example 5: C54H12
P-52.2.6.3 Seco(C60-Ih)[5,6]fullerenes and seco(C70-D5h(6))[5,6]fullerenes.
Example 1: C70H16
Example 2: C60H16
P-52.2.6.4. Seconorfullerenes
Example 1: C54H10
Example 2: C30H14
 |  | |
| (I) | (II) | |
| 3,2,13,12-(epihexa[1,3,5]trien[1,3,4,6]tetrayl)-6,9-methenocycloundeca[1,11,10-cd:6,7,8-c′d′]diindene (I) (PIN) [not 57,58:52,60-diseco-1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,25,26,29,30,33,34,37,38-triacontanor(C60-Ih)[5,6]fullerene (II)] | ||
Explanation: The preferred IUPAC name for this fullerene fragment is a systematic bridged fused ring name because it has 30 carbon atoms and only 7 five- and six-membered rings.
Example 3: C40H20
 |  | |
| (I) | (II) | |
| 2,15:3,14-dimethenoindeno[5′′,4′′:6′,7′]cyclododeca[1′,2′:4,5]indeno[1,2-b]anthracene (I) (PIN) [not 1,6:3,4-diseco-10,11,12,13,14,19,20,21,27,28,29,30,31,32,33,38,39,40,41,42,43,48,49,50,51,52,57,58,59,60-triacontanor(C70-D5h(6))[5,6]fullerene (II)] | ||
Explanation: The preferred IUPAC name for this fullerene fragment is a systematic fused ring name because it contains 40 carbon atoms but only eight five- and six-membered rings.
P-52.2.7 Preferred IUPAC names and numbering for ring assemblies
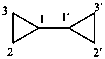
P-52.2.7.1 Preferred IUPAC names for assemblies of two or more identical cyclic systems joined by a single bond are formed using the names of parent hydrides rather than the names of substituent groups, except for biphenyl and polyphenyl assemblies, for which the name benzene is never used. For two-component assemblies, locants of one ring are unprimed; locants of the second ring are primed. Locants, including the locants 1 and 1′, are necessary in preferred IUPAC names to indicate points of attachment of rings or ring systems (see P-28.2.1).
Examples:
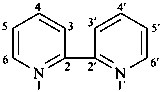
2,2′-bipyridine (PIN)
2,2′-bipyridyl
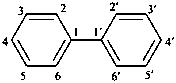
1,1′-biphenyl (PIN)
biphenyl
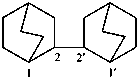
2,2′-bi(bicyclo[2.2.2]octane) (PIN)
2,2′-bi(bicyclo[2.2.2]octanyl)
A new numbering system is now recommended for preferred IUPAC names of ring assemblies with more than two rings or ring systems; it consists of composite locants, for example, 12. The previously used locant system for ring assemblies with more than two rings or ring systems using serially primed locants (refs. 1 and 2) may be used in general nomenclature.
Examples:
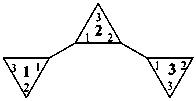 | 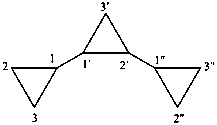 | |
| 11,21:22,31-tercyclopropane (PIN) | 1,1′:2′,1′′-tercyclopropane |
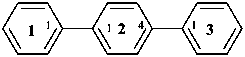
11,21:24,31-terphenyl (PIN)
1,1′:4′,1′′-terphenyl
(not p-terphenyl)
Phane names are preferred IUPAC names rather than ring assembly names when seven or more rings or ring systems are present.
Examples:

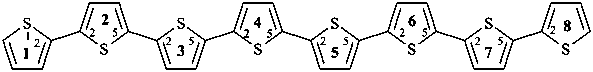
1,8(2),2,3,4,5,6,7(2,5)-octathiophenaoctaphane (PIN)
12,22:25,32:35,42:45,52:55,62:65,72,75,82-octithiophene
2,2′:5′,2′′:5′′,2′′′:5′′′,2′′′ ′:5′′′ ′,2′′′ ′′:5′′′ ′′,2′′′ ′′′:5′′′ ′′′,2′′′ ′′′ ′-octithiophene
Within the same heteroatom class and for the same number of characteristic groups cited as the principal characteristic group, a ring is always selected as the parent hydride to construct a preferred IUPAC name. In general nomenclature, a ring or a chain can be the parent hydride (see P-44.1.2.2).
Examples:
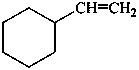
(a) ethenylcyclohexane (PIN) (ring preferred to chain)
(b) cyclohexylethene (emphasizes unsaturation)
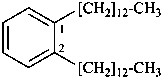
1,2-di(tridecyl)benzene (PIN) (ring preferred to chain)
[not 1,1′-(1,2-phenylene)di(tridecane);
multiplication of acyclic hydrocarbons is not allowed]
A certain number of retained names of parent hydrides are still recommended. The names methane, ethane, propane, and butane have been used since the beginning of systematic nomenclature. Names of cyclic mancude compounds are retained as components of fusion nomenclature; they are also used as preferred IUPAC names to name their derivatives and in general nomenclature.
An important aspect of these retained names is their substitutability; as parent compounds, they accommodate without restriction substituent groups cited as suffixes and prefixes. A few are limited in their capacity to be substituted; amongst them are the substituted benzenes ‘toluene’, ‘xylene’, and ‘mesitylene’. Some retained names are no longer recommended, for example ‘cumene’ and ‘cymene’.
For preferred retained IUPAC names of parent hydrides, see P-21.1.1 and P-21.1.2 for acyclic parent hydrides, P-22.1 and P-22.2 for monocyclic parent hydrides and P-25.1 and P-25.2 for polycyclic parent hydrides.
P-54 SELECTING THE PREFERRED METHOD FOR MODIFYING THE DEGREE OF HYDROGENATION
P-54.1 METHODS FOR MODIFYING THE DEGREE OF HYDROGENATION OF PARENT HYDRIDES
There are three methods for modifying the degree of hydrogenation for parent hydrides:
(1) by changing the ending ‘ane’ in acyclic parent hydrides to ‘ene’ and ‘yne’;(2) by using ‘hydro’ prefixes to saturate one or more double bonds in mancude compounds;
(3) by using ‘dehydro’ prefixes to introduce triple bonds in mancude compounds (see P-54.4).
Systematic IUPAC names and retained names of parent hydrides may be modified in the same way or in different ways to generate preferred IUPAC names.
P-54.2 UNSATURATED MONOCYCLIC CARBOCYCLES
Two methods are used for modifying the degree of hydrogenation of monocyclic carbocycles:
(1) by using the endings ‘ene’ and ‘yne’;Method (1) generates preferred IUPAC names:(2) by using the parent name ‘annulene’.
Examples:
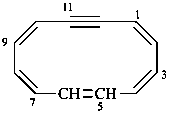
(1Z,3E,5Z,7E,9Z)-cyclododeca-1,3,5,7,9-pentaen-11-yne (PIN)
1,2-didehydro[12]annulene (numbering shown)
When assemblies of otherwise identical rings contain both mancude and saturated rings, the use of hydro prefixes is preferred, except in the case of a two ring assembly consisting of one benzene ring and a cyclohexane ring. However, when the requirements for the formation of phane names are met (see P-52.2.5.1), phane names are preferred IUPAC names.
Examples:
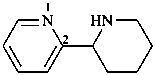
1,2,3,4,5,6-hexahydro-2,2′-bipyridine (PIN)
2-(piperidin-2-yl)pyridine

1(1),4(1,4)-dibenzena-2,3,5,6(1,4),7(1)-pentacyclohexanaheptaphane (PIN)
14-[4-(4′-phenyl[1,1′-bi(cyclohexan)]-4-yl)phenyl]-11,21:24,31-tercyclohexane
In these recommendations, the prefixes ‘hydro’ and ‘dehydro’ are detachable, but are not included in the category of alphabetized detachable prefixes (see P-14.4; see also P-15.1.5.2, P-31.2, P-58.2), which is a change from recommendations in earlier editions (ref. 1, 2) where they were alphabetized along with substituent prefixes. When along with the endings ‘ene’ and ‘yne’ they are used to modify parent hydrides, they are regulated by the principle of lowest locants, in accord with the numbering of the parent hydride and after priority has been given to indicated hydrogen, added indicated hydrogen, and suffixes, when present, as specified in the general rules for numbering (P-14.4).
P-54.4.1 Hantzsch-Widman heteromonocycles
Preferred IUPAC names for Hantzsch-Widman rings correspond to either fully unsaturated or fully saturated compounds (see P-22.2.2.1.1). ‘Hydro’ prefixes added to names of fully unsaturated Hantzsch-Widman rings lead to preferred IUPAC names for partially unsaturated rings; names containing the ‘dehydro’ prefix are allowed only in general nomenclature.
The final ‘e’ in Hantzsch-Widman names is required in preferred IUPAC names; it is still optional in general nomenclature. In the 1979 Rules (ref. 1), the final ‘e’ of a Hantzsch-Widman name was omitted when there was no nitrogen in the ring; in the 1993 Guide (ref. 2) this omission was made optional.
Examples:
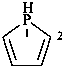
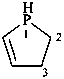
2,3-dihydro-1H-phosphole (PIN)
2,3-didehydrophospholane
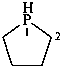
phospholane (PIN)
Preferred IUPAC names for saturated heteromonocyclic compounds are either Hantzsch-Widman names described in P-22.2.2.1.1 or retained names given in Table 2.3. Names of saturated rings derived by using hydro prefixes with Hantzsch-Widman names (see P-54.4.1) and retained names by adding the maximum of hydro prefixes, or ‘cyclo’ names described in P-22.2.5, are not preferred IUPAC names, but they may be used in general nomenclature.
Examples:
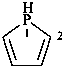
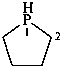
phospholane (PIN)
2,3,4,5-tetrahydro-1H-phosphole
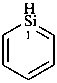
siline (PIN)
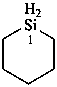
silinane (PIN)
hexahydrosiline
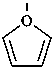
furan (PIN)
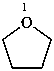
oxolane (PIN)
tetrahydrofuran
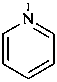
pyridine (PIN)
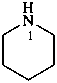
piperidine (PIN)
hexahydropyridine
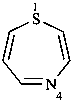
1,4-thiazepine (PIN)
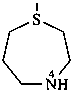
1,4-thiazepane (PIN)
hexahydro-1,4-thiazepine
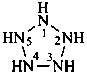
cylopentaazane
pentazolidine (preselected name; see P-22.2.2.1.2)
P-54.4.3.1 Retained fusion names are used for the fully unsaturated compounds (see P-25); they are the preferred IUPAC names. Preferred IUPAC names for the partially saturated and fully saturated compounds are formed by using ‘hydro’ prefixes. Preferred IUPAC names for partially saturated and mancude ring assemblies are formed in the same way.
In these recommendations, the prefix ‘hydro’ is detachable, but is not included in the category of alphabetized detachable prefixes (see P-14.4; see also P-15.1.5.2, P-31.2, P-58.2), which is a change from recommendations in earlier editions (ref. 1, 2) where it was alphabetized along with prefixes. When along with the endings ‘ene’ and ‘yne’ it is used to modify parent hydrides, it is regulated by the principle of lowest locants, in accord with the numbering of the parent hydride and after priority has been given to indicated hydrogen, added indicated hydrogen, and suffixes, when present, as specified in the general rules for numbering (P-14.4).
Examples:
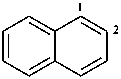
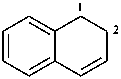
1,2-dihydronaphthalene (PIN)
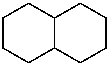
decahydronaphthalene (PIN)
bicyclo[4.4.0]decane
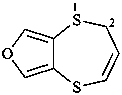
2H-[1,4]dithiepino[2,3-c]furan (PIN)
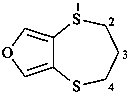
3,4-dihydro-2H-[1,4]dithiepino[2,3-c]furan (PIN)
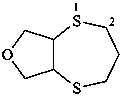
hexahydro-2H-[1,4]dithiepino[2,3-c]furan (PIN)
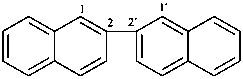
2,2′-binaphthalene (PIN)
2,2′-binaphthyl
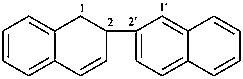
1,2-dihydro-2,2′-binaphthalene (PIN)
1,2-dihydro-2,2′-binaphthyl
1,2-dihydro-2-(naphthalen-2-yl)naphthalene
Example:
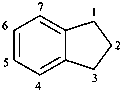
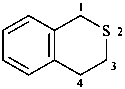
isothiochroman
3,4-dihydro-1H-2-benzothiopyran (PIN)
‘Dehydro’ prefixes are used to generate preferred IUPAC names for dehydrogenated mancude compounds. They may be used in general nomenclature to introduce double and triple bonds in saturated parent hydrides.
In these recommendations, the prefix ‘dehydro’ is detachable, but is not included in the category of alphabetized detachable prefixes (see P-14.4; see also P-15.1.5.2, P-31.2, P-58.2), which is a change from recommendations in earlier editions (ref. 1, 2) where it was alphabetized along with substituent prefixes. When along with the endings ‘ene’ and ‘yne’ it is used to modify parent hydrides it is regulated by the principle of lowest locants, in accord with the numbering of the parent hydride and after priority has been given to indicated hydrogen, added indicated hydrogen, and suffixes, when present, as specified in the general rules for numbering (P-14.4).
Examples:
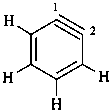
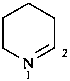
1,2-didehydropiperidine
2,3,4,5-tetrahydropyridine (PIN)
Trivial names retained for naming organic compounds are known as ‘retained names’. Parent hydrides and functional parent compounds may have retained names. The number of parent hydrides having retained names has been kept almost unchanged through the years. The main reason is that the aliphatic ones methane, ethane, propane and butane have been in use since the Geneva Convention; most of the cyclic parent hydrides are used as components in systematic fusion nomenclature as discussed in Section P-52. The situation regarding functional parent compounds is different. Their number was sharply reduced in the 1979 Rules, and reduced still further in the 1993 Recommendations. In these recommendations, their usage as preferred IUPAC names has been sharply limited. All retained names in the 1993 list can be used in general and specialized nomenclature. Two distinct classes are thus recognized.
(1) Retained names used as preferred IUPAC names.A further classification regarding substitution was established in the 1993 Recommendations. Structures corresponding to retained names could be substituted without restriction, substituted in a limited way, or simply could not be substituted at all. This issue is discussed in P-15.1.8.(2) Retained names recommended for general nomenclature.
In the context of preferred IUPAC names, most parent hydrides having retained names recommended as preferred IUPAC names are fully substitutable and most functional parent compounds having retained names also recommended as preferred IUPAC names can be substituted, albeit in the limited way imposed by the presence of a characteristic group and the seniority of classes. Exceptions are known, when no substitution is allowed.
Retained names recommended for general and specialized nomenclature must be used as in the past. Rules about substitution of corresponding structures are less strict and traditional IUPAC nomenclature can still be applied in all its diversity and adaptability. In this Section, preferred IUPAC names only will be discussed.
Organic functional parent compounds having retained names used as preferred IUPAC names are all listed in P-34.1. Substitution is allowed on all structures except anisole and tert-butoxy, and formic acid and formyl group are substitutable with limitations.
For inorganic parent compounds, see P-67.1.2 and P-67.2.1. Organic functional parent compounds for general nomenclature, see P-34.1.3.
P-56 SELECTING THE PREFERRED SUFFIX FOR THE PRINCIPAL CHARACTERISTIC GROUP
Suffixes have always been considered as the most unique elements of the formation of names. In the past, some suffixes have been discarded and replaced by new ones. The following suffixes have been introduced or modified in these recommendations.
P-56.1 THE SUFFIX ‘PEROXOL’, FOR –OOH
It is now recommended to use the sufix ‘peroxol’ to provide substitutive names for hydroperoxides. Such names are preferred to those generated by functional class nomenclature.
The suffix ‘peroxol’ for –OOH is now adopted to name hydroperoxides, which in previous recommendations were named by functional class nomenclature.
Example:
The suffix ‘sulfenic acid’ and its chalcogen analogues were discarded in the 1993 recommendations (ref. 2). In these recommendations, these suffixes are replaced by the new suffixes, ‘SO-thioperoxol’, ‘SeO-selenoperoxol’, ‘dithioperoxol’, ‘TeS-tellurothioperoxol’, ‘diselenoperoxol’, ‘SeTe-selenotelluroperoxol’ and ‘TeSe-telluroselenoperoxol’ (see P-63.4.2.1).
The suffixes SO-thioperoxol and its chalcogen analogues are now introduced to replace the suffix ‘sulfenic acid’ and its chalcogen analogues that were discarded in the 1993 recommendations.
Examples:
C6H5-SeSe-H
benzenediselenoperoxol (PIN)
(not benzeneselenoselenic acid)
The suffixes ‘amidine’ and ‘carboxamidine’, for –C(=NH)-NH2 and –(C)(=NH)-NH2, are no longer recommended. They are replaced by the new functional replacement suffixes ‘imidamide’ and ‘carboximidamide’ in preferred IUPAC names (see P-66.4.1).
Examples:
C6H11-C(=NH)-NH2
cyclohexanecarboximidamide (PIN)
(no longer cyclohexanecarboxamidine)
Except for ‘methylene’, ‘ethylene’, and ‘phenylene’, the suffix ‘ylene’ previously used to describe divalent substituent groups in which the free valences do not form a double bond, i.e., –E– or E< was discarded in 1993 (ref. 2). Substituent groups in which the free valences form a double bond, i.e., E= were described by the suffix ‘ylidene’. The suffix ‘ylene’ was replaced by the suffixes ‘diyl’ to express the –E– or E< type of bonding, and ‘ylidene’ for E=, for example, ethane-1,2-diyl for –CH2-CH2– and ethylidene for H3C-CH=, respectively. However, the name ‘methylene’ is retained to describe the substituent group –CH2–; it is used in preferred IUPAC names rather than methanediyl. CAS still use the ‘ylene’ suffix to describe the ‘diyl’ and ‘ylidene’ types of bonds, especially ‘methylene’ for –CH2– and CH2=.
Examples:
| –CH2– | H2C= | |
| methylene (preferred prefix) (not methanediyl) | methylidene (preferred prefix) (formerly methylene) | |
| –CH2-CH2– | CH3-CH= | |
| ethane-1,2-diyl (preferred prefix) ethylene | ethylidene (preferred prefix) | |
| –SiH2– | H2Ge= | |
| silanediyl (preselected prefix) (not silylene, a name still used by CAS) | germylidene (preselected prefix) (not germylene, a name still used by CAS) | |
| –BH– | HB= | |
| boranediyl (preselected prefix) (not borylene, a name still used by CAS) | boranylidene (preselected prefix) (not borylene, a name still used by CAS) | |
| –SbH– | HSb= | |
| stibanediyl (preselected prefix) (not stibinediyl) (not stibylene, a name still used by CAS) | stibanylidene (preselected prefix) (not stibinylidene) (not stibylene, a name still used by CAS) |
–NH-CO-NH–
carbonylbis(azanediyl) [preferred prefix,
a name used in multiplicative nomenclature; (see P-66.1.6.1.1.3)]
(not carbonyldiimino,
a name still used by CAS)
(not ureylene)
Preferred prefixes for substituent groups are considered here in three different sections. A comprehensive list is provided in Appendix 2.
All substituent groups are named systematically using substitutive nomenclature. Some names are retained; they are important because they do have priority over systematic substitutive names.
P-57.1 Prefixes derived from parent hydridesP-57.1 PREFIXES DERIVED FROM PARENT HYDRIDES
P-57.2 Prefixes derived from characteristic (functional) groups
P-57.3 Prefixes derived from functional parent compounds
P-57.4 Construction of linear compound and/or complex substituent prefixes.
P-57.1.1 Prefixes derived from mononuclear and acyclic parent hydrides
P-57.1.1.1 When the free valences are in position 1 of substituent groups derived from mononuclear hydrides carbon, silicon, germanium, tin and lead and from acyclic hydrocarbons, preferred prefixes are of the ‘alkyl type’ according to P-29.2; preferred prefixes are of the ‘alkanyl type’ for all mononuclear hydrides other than those mentioned above and for saturated substituent groups when the free valences are not in position 1.
Examples:
PH2–
phosphanyl (preselected prefix)
SiH3–
silyl (preselected prefix)
silanyl
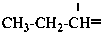
propylidene (preferred prefix)
propan-1-ylidene
CH2=
methylidene (preferred prefix)
methanylidene
CH3-C≡
ethylidyne (preferred prefix)
ethanylidyne
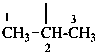
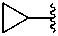
propan-2-yl (preferred prefix)
1-methylethyl
isopropyl
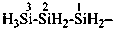
trisilan-1-yl (preselected prefix)
P-57.1.2 The following retained names are used as preferred prefixes for which no substitution is recommended:
Examples:
C6H5-CH2–
benzyl (preferred prefix)
phenylmethyl
C6H5-CH=
benzylidene (preferred prefix)
phenylmethylidene
C6H5-C≡
benzylidyne (preferred prefix)
For preferred prefixes, the names ‘benzyl’, ‘benzylidene’, and ‘benzylidyne’ cannot be substituted. Previously, in the 1993 Guide (ref. 2), they could only be substituted on the ring. However, for general nomenclature restricted substitution is permitted (see P-29.6.2.1).
P-57.1.3 Retained prefixes recommended only for general nomenclature
The prefix ‘ethylene’, for –H2C-CH2–, is recommended, with unlimited substitution, only for general nomenclature (P-29.6.2.3).
Isopropyl for (CH3)2CH–, isopropylidene for (CH3)2C=, and trityl for (C6H5)3C– are retained as prefixes only for use in general nomenclature but no substitution of any kind is allowed (see P-29.6.2.2).
P-57.1.4 Retained prefixes no longer recommended
The retained names phenethyl (2-phenylethyl) for C6H5-CH2-CH2–; benzhydryl (diphenylmethyl), for (C6H5)2CH–; isobutyl (2-methylpropyl) for (CH3)2CH-CH2–; sec-butyl (butan-2-yl, 1-methylpropyl) for CH3-CH2-CH(CH3)–; isopentyl (3-methylbutyl) for (CH3)2CH-CH2-CH2–; tert-pentyl (2-methylbutan-2-yl, 1,1-dimethylpropyl) for CH3-CH2-C(CH3)2–; and neopentyl (2,2-dimethylpropyl) for (CH3)3C-CH2–; are no longer recommended; the first name in parentheses is the preferred prefix name.
P-57.1.5 Prefixes derived from cyclic parent hydrides
P-57.1.5.1 Preferred prefixes derived from cycloalkanes are of the ‘cycloalkyl type’ (see P-29.2); preferred prefixes derived from cyclic compounds other that cycloalkanes are all of the ‘alkanyl type’ described above in P-57.1.1.1.
Examples:
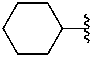
cyclohexyl (preferred prefix)
cyclohexanyl
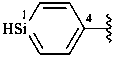
silin-4-yl (preferred prefix)
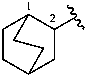
bicyclo[2.2.2]octan-2-yl (preferred prefix)
bicyclo[2.2.2]oct-2-yl
The two following prefixes are retained as preferred prefixes with unlimited substitution:
1,4-phenylene (also 1,2- and 1,3-isomers)
(preferred prefixes)
The following retained prefixes are recommended for only general nomenclature, with unlimited substitution except for tolyl for which no substitution is allowed:
2-anthryl (also 1- and 9-isomers)
anthracen-2-yl (preferred prefixes)
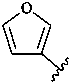
3-furyl (also 2-isomer)
furan-3-yl (also 2-isomer;
preferred prefixes)
7-isoquinolyl (also 1-, 3-, 4-, 5-, 6- and 8-isomers)
isoquinolin-7-yl (also 1-, 3-, 4-, 5-, 6- and 8-isomers,
preferred prefixes)
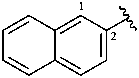
2-naphthyl (also 1-isomer)
naphthalen-2-yl (also 1-isomer;
preferred prefixes)
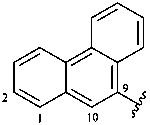
9-phenanthryl (also 1-, 2-, 3- and 4-isomers)
phenanthren-9-yl (also 1-, 2-, 3- and 4-isomers,
preferred prefixes)
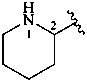
2-piperidyl (also 3- and 4-isomers)
piperidin-2-yl (also 3- and 4-isomers;
preferred prefixes)
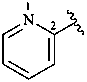
2-pyridyl (also 3- and 4-isomers)
pyridin-2-yl (also 3- and 4-isomers;
preferred prefixes)
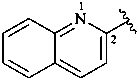
2-quinolyl (also 3-, 4-, 5-, 6-, 7- and 8-isomers)
quinolin-2-yl (also 3-, 4-, 5-, 6-, 7- and 8-isomers;
preferred prefixes)
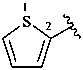
2-thienyl (also 3-isomer)
thiophen-2-yl (also 3-isomer;
preferred prefixes)
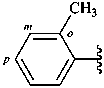
o-tolyl (also m- and p-isomers;
no substitution allowed)
2-methylphenyl (also 3- and 4-isomers;
preferred prefixes)
The retained prefixes furfuryl (2 isomer only) and thenyl (2 isomer only) are no longer recommended (see P-29.6.3)
P-57.1.6 Prefixes derived from parent hydrides with modified degrees of hydrogenation
P-57.1.6.1 All preferred prefixes derived from parent hydrides whose degree of hydrogenation has been modified are formed systematically according to rules discussed in P-32. There is a choice when the free valences are in position 1 or at any position on the chain: preferred prefixes are given to the less substituted chain (see example 3 below, and many examples in P-32.1.1).
Examples:
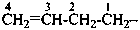
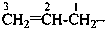
prop-2-en-1-yl (preferred prefix)
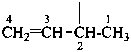
but-3-en-2-yl (preferred prefix)
1-methylprop-2-en-1-yl
bicyclo[2.2.2]oct-5-en-2-yl (preferred prefix)
spiro[4.5]deca-1,9-dien-6-ylidene (preferred prefix)
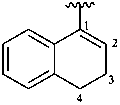
3,4-dihydronaphthalen-1-yl (preferred prefix)
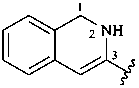
1,2-dihydroisoquinolin-3-yl (preferred prefix)
(1) In acyclic prefixes, the longest chain is chosen as the principal chain;
In these recommendations, a major change in the naming of substituents derived from unsaturated acyclic compounds is adopted in which the longest chain is chosen as the parent chain regardless of the number or type of multiple bonds.
Examples:
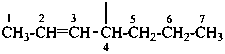 | 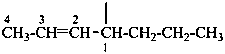 | |
| hept-2-en-4-yl [alkanyl type; see P-29.2 method (2)] (preferred prefix) | 1-propylbut-2-en-1-yl [alkyl type; see P-29.2 method (1)] | |
(2) In acyclic prefixes derived from alkanes modified by skeletal replacement (‘a’) nomenclature, the ‘a’ prefixes have seniority over suffixes, such as ‘yl’ and ‘ylidene’.
Fixed numbering for heteroacyclic parent structures named by skeletal replacement (‘a’) nomenclature is a major change to Rule C-0.6 (ref. 1) where principal characteristic groups and free valence were preferred over heteroatoms for low locants.
Example:
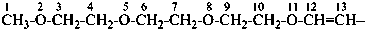
There are no retained prefixes derived from parent hydrides with a modified degree of hydrogenation recommended as preferred prefixes. The retained prefixes vinyl (ethenyl), for CH2=CH–; vinylidene (ethenylidene), for CH2=C=; allyl (prop-2-en-1-yl), for 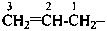 ; allylidene (prop-2-en-1-ylidene), for
; allylidene (prop-2-en-1-ylidene), for 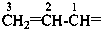 ; and allylidyne (prop-2-en-1-ylidyne) for CH2=CH-C≡; are retained but only for general nomenclature (see P-32.3). Substitution is allowed, but not by alkyl or any other group that extends the carbon chain or by characteristic groups expressed by suffixes. The preferred prefixes are given in parentheses.
; and allylidyne (prop-2-en-1-ylidyne) for CH2=CH-C≡; are retained but only for general nomenclature (see P-32.3). Substitution is allowed, but not by alkyl or any other group that extends the carbon chain or by characteristic groups expressed by suffixes. The preferred prefixes are given in parentheses.
The prefix isopropenyl (prop-1-en-2-yl), for CH2=C(CH3)–, is a retained prefix but is not used as a preferred prefix. It is acceptable for general use but no substitution is allowed. The preferred prefix is given in parentheses.
The prefixes indan-2-yl, indolin-2-yl, isoindolin-2-yl, chroman-2-yl and isochroman-2-yl, as well as other isomers, are recommended for general nomenclature only, with unlimited substitution (see Table 3.2).
P-57.2 PREFIXES DERIVED FROM CHARACTERISTIC (FUNCTIONAL) GROUPS
Names of prefixes derived from characteristic groups are either retained names or are systematically formed by substitutive nomenclature. Retained prefixes are described in P-35.2.1 and P-35.2.3. Substitutive systematic prefixes are formed by the general methodology described for prefixes derived from parent hydrides (see P-57.1.1). In fact, prefixes derived from characteristic groups are those derived from parent hydrides of Groups 17, 16 and from azane in Group 15; they are discussed in P-35.2.2.
P-57.3 PREFIXES DERIVED FROM ORGANIC FUNCTIONAL PARENT COMPOUNDS
Names of prefixes derived from functional parent compounds are either retained prefixes or are systematically formed by substitutive nomenclature. Retained prefixes corresponding to functional parent compounds used as preferred prefixes are described in P-34.2. Retained prefixes derived from functional compounds that can only be used in general nomenclature are described in Chapter P-6 in Sections related to specific classes. Appendix 2 contains all prefixes derived from functional parent hydrides.
Examples:
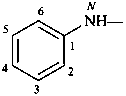
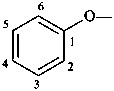
phenoxy (preferred prefix)
(a retained simple prefix derived from phenol;
substitution allowed; see P-63.2.2.2)
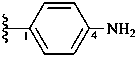
4-aminophenyl (preferred prefix)
(a systematic compound prefix)
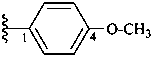
4-methoxyphenyl (preferred prefix)
(a systematic compound prefix)
H-CO–
formyl (preferred prefix,
see P-65.1.7.2.1)
CH3-CO–
acetyl (preferred prefix,
see P-65.1.7.2.1)
Linear compound and complex prefixes are constructed in a stepwise manner by working backwards component by component from the free valence. At each step, when a choice is possible, the largest nomenclaturally significant component is chosen.
Contracted prefixes, such as methoxy are considered in their systematic uncontracted form, i.e. methyloxy.
Examples:
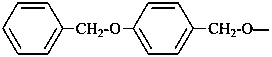
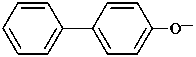
P-58 SELECTION OF PREFERRED IUPAC NAMES
P-58.1 INTRODUCTION.
Section P-45 contains the hierarchical rules for choosing a preferred IUPAC name based on an order of seniority (see P-44) for determining the one and only parent structure. Preferred IUPAC names are generated under the condition that the name of the parent structure and the names of all or part of components are preferred IUPAC names. When this condition is not fulfilled and when the names of components are acceptable for general nomenclature, the resulting names of the compounds are acceptable only for general nomenclature.
Examples:
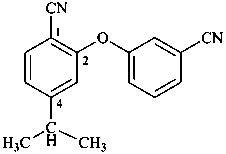
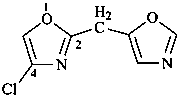
P-58.2.1 Indicated hydrogen (see also P-14.7.1).
Indicated hydrogen, if needed, is always cited at the front of the name of a spiro ring system, a bridged ring system, or a ring assembly, which is a change from its position in previous recommendations for bridged ring systems and ring assemblies where it was kept with the name of the individual ring.
P-58.2.1.1 In many ‘mancude’ rings (see P-22.2.2.1.4), fused ring systems (see P-25.7.1.3), bridged fused ring systems (see P-25.7.1.3.3), spiro ring systems (see P-24.3), or ring assemblies (see P-28.2.3), it is necessary to specify hydrogen atoms of ring atoms that are attached only by single bonds to adjacent ring atoms in order that the principles of substitutive nomenclature can be used to describe characteristic groups, free valences, or ionic sites, i.e, to accommodate characteristic groups, free valences, or ionic sites. This is accomplished by specifying the presence of a hydrogen atom at such positions by the citation of an italicized capital ‘H’ preceded by an appropriate numerical locant cited at the front of the name; this indicator is called ‘indicated hydrogen’. Indicated hydrogen is often omitted for very common isomers or where there is no ambiguity in the name; however, in preferred IUPAC names indicated hydrogen must always be cited when present in the corresponding structure.
P-58.2.1.2 In parent hydrides, indicated hydrogen atoms (see P-14.7) are cited, if posible, at the lowest nonfusion peripheral atom (see P-25.0) of the ring or ring system consistent with the maximum number of noncumulative double bonds in accordance with P-25.7. Low locants are assigned to indicated hydrogen atoms when the degree of unsaturation is modified by using ‘hydro’ prefixes.
Examples:
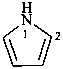
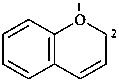
2H-1-benzopyran (PIN)
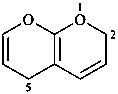
2H,5H-pyrano[2,3-b]pyran (PIN)
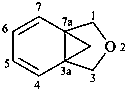
1H,3H-3a,7a-methano-2-benzofuran (PIN)
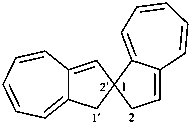
1′H,2H-1,2′-spirobi[azulene] (PIN)
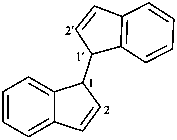
1H,1′H-1,1′-biindene (PIN)
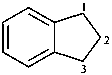
2,3-dihydro-1H-indene (PIN)
(not 1,3-dihydro-2H-indene;
nor 1,2-dihydro-3H-indene)
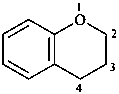
3,4-dihydro-2H-1-benzopyran (PIN)
(not 2,3-dihydro-4H-1-benzopyran;
although other indicated hydrogen combinations are possible,
this combination has the lowest possible locants that are structurally permissible for this compound)
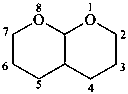
hexahydro-2H,5H-pyrano[2,3-b]pyran (PIN)
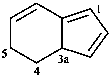
3a,5-dihydro-4H-indene (PIN)
(not 4,5-dihydro-3aH-indene)
A second type of indicated hydrogen describes hydrogen atoms attached to ring atoms that are attached to adjacent ring atoms by single bonds only as the consequence of the addition of a suffix describing a structural modification. This type of indicated hydrogen is called ‘added indicated hydrogen’ because it is added to the name as a result of an operation on the parent hydride which may or may not contain indicated hydrogen atoms. ‘Added indicated hydrogen’ is cited in parentheses after the locant of the structural feature to which it refers. This method is preferred over the use of nondetachable hydro prefixes (P-58.2.5) for preferred IUPAC names.
Note: Indicated hydrogen has been used in the manner described in P-58.2.1, above but applied after the introduction of a principal characteristic group. It significantly reduced the need for ‘added indicated hydrogen’. This method was developed at the Beilstein Institute and may be found in the Beilsteins Handbuch der Organischen Chemie, Springer Verlag, printed edition 1909-1959. It is not recommended for use in constructing IUPAC names but may be found in names in the literature.
P-58.2.2.1 General methodology
The presence of at least one hydrogen atom on a ring atom that is attached to adjacent ring atoms by single bonds only that results from the introduction of a principal characteristic group, a free valence, radical, or an ionic center into a mancude polycyclic system in the absence of, or lack of, sufficient hydrogen atoms, is cited by using the capital italic letter H following the locant of the ring atom for each such position. This ‘added indicated hydrogen’ designation is enclosed in parentheses and inserted into the name immediately following the locant(s) for the free valences, radical or ionic centers, or principal characteristic groups.
P-58.2.2.2 When there is a choice, ‘added indicated hydrogen’ positions are assigned to peripheral ring atoms, with the lowest locants consistent with the arrangement of double bonds in the compound as required. Low locants are assigned to ‘added indicated hydrogen’ atoms in the presence of hydro prefixes used to modify the degree of unsaturation.
Examples:
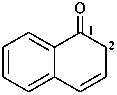
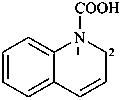
quinoline-1(2H)-carboxylic acid (PIN)
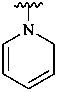
pyridin-1(2H)-yl (preferred prefix)
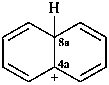
naphthalen-4a(8aH)-ylium (PIN)
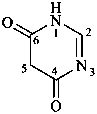
pyrimidine-4,6(1H,5H)-dione (PIN)
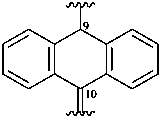
anthracen-9(10H)-yl-10-ylidene (preferred prefix)
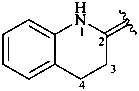
3,4-dihydroquinolin-2(1H)-ylidene (preferred prefix)
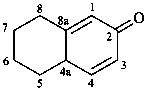
5,6,7,8-tetrahydronaphthalen-2(4aH)-one (PIN)
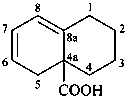
1,3,4,5-tetrahydronaphthalene-4a(2H)-carboxylic acid (PIN)
(a 4a(1H)-isomer is not consistent with the arrangement
of the double bonds in the mancude compound)
Examples:
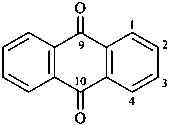
anthracene-9,10-dione (PIN)
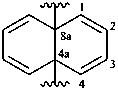
naphthalene-4a,8a-diyl (preferred prefix)
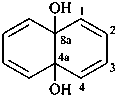
naphthalene-4a,8a-diol (PIN)
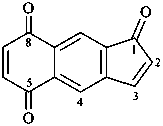
1H-cyclopenta[b]naphthalene-1,5,8-trione (PIN)
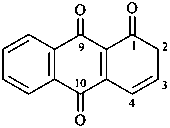
anthracene-1,9,10(2H)-trione (PIN)
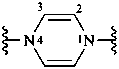
pyrazine-1,4-diyl (preferred prefix)
P-58.2.3.1 Indicated hydrogen is cited at any position of a ring system in order to accommodate principal characteristic groups or free valences expressed as suffixes, provided that there are an equal or greater number of indicated hydrogen atoms available to accommodate all of the principal characteristic groups or free valences.
P-58.2.3.1.1 When there are an equal number of indicated hydrogen atoms and principal characteristic groups or free valences to be accommodated, the indicated hydrogen atoms are placed at peripheral atoms that will accommodate these principal characteristic groups or free valences. Locants for hydro prefixes are those of the saturated positions.
Examples:
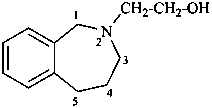
2-(1,3,4,5-tetrahydro-2H-2-benzazepin-2-yl)ethan-1-ol (PIN)
1,3,4,5-tetrahydro-2H-2-benzazepine-2-ethanol
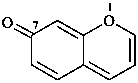
7H-1-benzopyran-7-one (PIN)
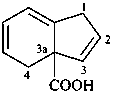
1,4-dihydro-3aH-indene-3a-carboxylic acid (PIN)
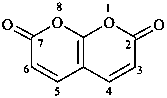
2H,7H-pyrano[2,3-b]pyran-2,7-dione (PIN)
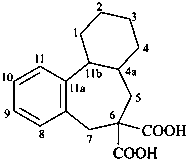
1,2,3,4,4a,5,7,11b-octahydro-6H-dibenzo[a,c][7]annulene-6,6-dicarboxylic acid (PIN)
[not 1,2,3,4,4a,5,7,11b-octahydro-6H-dibenzo[a,c]cycloheptene-6,6-dicarboxylic acid]
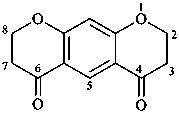
2,3,7,8-tetrahydro-4H,6H-benzo[1,2-b:5,4-b′]dipyran-4,6-dione (PIN)
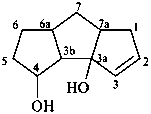
1,3b,4,5,6,6a,7,7a-octahydro-3aH-cyclopenta[a]pentalene-3a,4-diol (PIN)
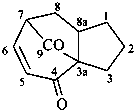
1,2,3,7,8,8a-hexahydro-4H-3a,7-methanoazulene-4,9-dione (PIN)
Examples:
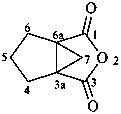
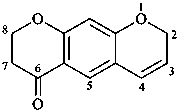
7,8-dihydro-2H,6H-benzo[1,2-b:5,4-b′]dipyran-6-one (PIN)
(not 2,3-dihydro-4H,8H-benzo[1,2-b:5,4-b′]dipyran-4-one;
‘2H,6H’ is lower than ‘4H,8H’)
(not 6,7-dihydro-2H,8H-benzo[1,2-b:5,4-b′]dipyran-4(3H)-one;
‘2H,6H’ is lower than ‘2H,8H’)
(1) at least one of the indicated hydrogen atoms is assigned to a nonfusion peripheral atom having the lowest locants consistent with the mancude system of double bonds as established in P-58.2.1.2.Examples:(2) other indicated hydrogen atoms are assigned to other positions that accommodate characteristic groups or free valences.
(3) principal characteristic groups or free valences that cannot be accommodated in ways described in (1) and (2) are accommodated by using ‘added indicated hydrogen atoms’ (see P-58.2.2).
(4) indicated hydrogen atoms that cannot be used to accommodate principal characteristic groups or free valences has seniority over ‘added indicated hydrogen’ for lower locants.
1H-cyclopenta[a]naphthalene-1,2(3H)-dione (PIN)
(not 3H-cyclopenta[a]naphthalene-1,2-dione)
P-58.2.3.1.4 When the indicated hydrogen atoms of a parent structure cannot be used to accommodate all of the principal characteristic groups of the structure, the rules described in P-58.2.3.1.3 are applied
Examples:
P-58.2.4 Prefix nomenclature
After the introduction of indicated and ‘added indicated hydrogen’ atoms, all substituent groups not expressed as suffixes are cited as prefixes.
Examples:
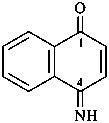
4-iminonaphthalen-1(4H)-one (PIN)
An alternative method to the ‘added indicated hydrogen’ method for accommodation of principal characteristic groups, free valences, radicals, or ionic centers at positions of mancude parent hydrides where a sufficient number of hydrogen atoms for the operation of the basic principles of substitutive nomenclature are not present is to derive them from a suitable hydrogenated derivative of the parent ring system.
Rule C-16.11 in the 1979 edition of the IUPAC Nomenclature of Organic Chemistry (ref. 1) allows for hydro prefixes to be either nondetachable, i.e., they must always be cited directly in front of the name of a fully unsaturated parent structure, thus creating a parent hydride separate and distinct from the fully unsaturated analogue, or detachable, i.e., cited as prefixes in front of the name of a fully unsaturated parent structure, but alphabetized among any substituent prefixes that may also be present. The 1993 Guide (ref. 1) formalized the nondetachable method. In these recommendations, the use of nondetachable hydro prefixes is not used in preferred IUPAC names, but may be used in general nomenclature. This method often leads to differences in numbering of the parent structure (see fourth example, below).
Examples:
1,4-dihydronaphthalene-1,4-diylidene
naphthalene-1,4-diylidene (preferred prefix;
see P-58.2.2.3)
1,2,3,4-tetrahydronaphthalene-1,4-dione
2,3-dihydronaphthalene-1,4-dione (PIN;
see P-58.2.2.3)
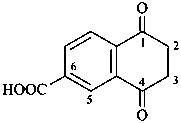
1,4-dioxo-1,2,3,4-tetrahydronaphthalene-6-carboxylic acid
(numbering shown; nondetachable hydro prefixes are part of the parent hydride
and have precedence over the principal characteristic group for low numbering)
5,8-dioxo-5,6,7,8-tetrahydronaphthalene-2-carboxylic acid (PIN)
(detachable but nonalphabetized hydro prefixes do not have precedence
over the principal characteristic group for low numbering,
but has precedence over other detachable prefixes).
P-58.3.1 Preselected names for unbranched homogeneous heteroacyclic parent hydrides other than boron hydrides are described in P-21.2.2. Potential functionality of terminal groups, such as –NH2, –SH, or –OH, attached to parent chains of the same heteroatom are ignored; they simply extend the chain. For boron chains see P-68.1.1.2. For chains involving chalcogen atoms, see P-68.4.
P-58.3.2 When one or more atoms of a homogeneous heteroatomic chain can be expressed by a principal characteristic group, a functional parent compound, or a compulsory prefix, the principal characteristic group or compulsory prefix group is expressed. Hence, an acyclic homogeneous heterocyclic chain may be broken in order to recognize a senior function as a principle characteristic groups, functional parent compound, or compulsory prefix.
Examples:
H2N-NH-NH-NH-CO-C6H5
N-(triazan-1-yl)benzamide (PIN)
[not phenyl(tetraazan-1-yl)methanone;
nor 1-benzoyltetraazane]
H2N-NH-NH-NH-COOH
tetraazane-1-carboxylic acid (PIN)
[not (triazan-1-yl)carbamic acid;
the carboxylic acid is senior to the carbonic acid derivative]
CH3-NH-N(COOH)-NH-CH3
1,3-dimethyltriazane-2-carboxylic acid (PIN)
(not bis(methylamino)carbamic acid;
the carboxylic acid is senior to the carbonic acid derivative)
(CH3)2P-P(OH)-P(CH3)2
bis(dimethylphosphanyl)phosphinous acid (PIN)
(not 1,1,3,3-tetramethyltriphosphan-2-ol)
H2N-NO
nitrous amide (preselected name)
(not hydrazinone; (see P-61.6)
H2N-NH-NH-NCO
1-isocyanatotriazane (PIN)
[not 1-(oxomethylidene)tetraazane]
C6H5-CO-NH-NH-NH-NH-CO-C6H5
N,N′-(hydrazine-1,2-diyl)dibenzamide (PIN)
OCN-NH-NO2
N-isocyanatonitramide (PIN)
HS-S-S-S-CO-C6H5
phenyl(tetrasulfanyl)methanone (PIN)
[not trisulfanyl benzenecarbothioate;
pseudoesters are not recognized when the alcoholic component
is a chalcogen atom (see P-65.6.3.4.2 and P-68.4.2.4)]
P-59.0 IntroductionP-59.0 INTRODUCTION
P-59.1 General methodology
P-59.2 Examples illustrating the methodology
This Section describes the procedure for the systematic formation of a preferred IUPAC name for an organic compound. This procedure can also be followed for generation of names for general nomenclature.
P-59.1 GENERAL METHODOLOGY
The procedure for formation of a preferred systematic name for an organic compound involves a number of steps outlined in this and in the following subsections, to be taken as far as they are applicable in the following order.
P-59.1.1 From the nature of the compound, determine the type(s) of nomenclature (see P-15) and operations (see P-13) to be used. Although the type of nomenclature called ‘substitutive nomenclature’ (see P-15.1) is the preferred type of nomenclature for generating preferred IUPAC names and for general nomenclature, other nomenclature types must be used when specified by specific classes of compounds rules:
(1) ‘functional class nomenclature’, for compounds such as esters and acid halides (see P-15.2 and P-51.2 for preferred IUPAC names and for general nomenclature);(2) ‘multiplicative nomenclature’ (see P-15.3, and P-51.3 for preferred IUPAC names and for general nomenclature);
(3) ‘skeletal replacement (‘a’) nomenclature’ (see P-15.4 and P-51.4 for preferred IUPAC names and for general nomenclature).
When configurations are expressed in the structure, select the type of nomenclature, substitutive or multiplicative nomenclature according to P-93.6, that will take them into consideration.
P-59.1.2 Determine the class to which the compound belongs and the characteristic group to be cited as the suffix as given in P-33, (if any) in accord with the seniority order of classes as indicated in P-41 or as a functional class name (see P-15.2). Only one kind of characteristic group (known as the principal group) can be cited as suffix or functional class name.
Suffixes listed in P-33 are used to generate both preferred IUPAC names and names in general nomenclature. The order of seniority of suffixes discussed in P-43 is mandatory for constructing preferred IUPAC names and in general nomenclature. All atoms or groups not so cited must be specified as substituent prefixes.
Radicals and ions are named using suffixes that have the unique property of being cumulative, both among themselves and in conjunction with certain suffixes that express characteristic groups (see Chapter P-7 for seniority order of radicals and ions to be used to generate preferred IUPAC names and in general nomenclature).
P-59.1.3 Select the parent hydride(s), including any appropriate nondetachable prefixes as described in Chapter P-2 and in P-52 for the selection of preferred IUPAC names, or functional parent compound as described in P-34 for preferred IUPAC names and in Chapter P-6 for various classes such as amines, alcohols, etc. which summarizes two aspects of the use of functional parent compounds in substitutive nomenclature, i.e., use as preferred IUPAC name or in general nomenclature and their substitutability. Determine the senior parent structure, either in the presence of a suffix describing the principal characteristic group or in the absence of any such suffix. All required descriptors for indicating changes from standard bonding number and isotopic modifications must be introduced at this stage.
P-59.1.4 Name the parent hydride, as described in P-59.1.3 and the principal characteristic group, if any, according to P-33, or the functional parent compound according to P-34 and Chapter P-6, using rules indicated in P-43 in order to take functional modifications into consideration. For ketones and imines, use Rules discussed in P-58.2 for generating preferred IUPAC names and for general nomenclature.
P-59.1.5 Determine suffixes and/or prefixes, in accordance with P-15.5, P-57, Chapter P-6, and Appendix 2 using appropriate multiplying prefixes (see P-14.2) and number the parent structure as far as possible using the general rule P-14.4.
P-59.1.6 Name substituent groups and characteristic groups not cited as principal characteristic groups as prefixes (alphabetized prefixes) in accordance with P-34 which summarizes the use of functional parent compounds in substitutive nomenclature, i.e., use as preferred IUPAC name or in general nomenclature, and P-15.1.8 for their substitutability and P-56 for preferred IUPAC names and for allowed names in general nomenclature, and complete the numbering of the structure, if necessary, according to the numbering rules for nomenclatural features given in P-14.4.
P-59.1.7 Assemble the components into a complete name, using rules described in Section P-14 (general rules) related to locants, numbering, alphanumerical order, indicated and added indicated hydrogen and aspects of name writing such as punctuation, enclosing marks, italicization, elision and addition of vowels, and primes as described in P-16.
P-59.1.8 Complete the name with all required descriptors for stereochemical features in accordance with rules described in Chapter P-9.
P-59.1.9 Characteristic groups
In substitutive nomenclature, some characteristic groups can be denoted either as suffixes or prefixes (see P-33 and P-35), but others only as prefixes (see Table 5.1). Functional class names differ in that a separate word (or a suffix in some languages) designating the name of a functional class is associated with a substituent group name describing the reminder of the structure.
Characteristic groups that can be cited as suffixes in substitutive nomenclature are not necessarily identical with groups designated by the name of a corresponding functional class when functional class names are formed (e.g., butanone and ethyl methyl ketone, where ‘one’ denotes =O and ‘ketone’ denotes –CO–).
The characteristic groups listed in Table 5.1 are always cited as prefixes to the name of the parent structure described in Chapter P-2. Multiplying prefixes (see P-14.2) and locants are added as necessary (see P-14.3).
| Characteristic group | Prefix | Characteristic group | Prefix |
| –Br | bromo | –IO1 | iodosyl |
| –BrO1 | bromosyl | –IO21 | iodyl |
| –BrO21 | bromyl | –IO31 | periodyl |
| –BrO31 | perbromyl | –O-R2,3 | alkoxy |
| –Cl | chloro | –O-O-R2,4 | alkylperoxy |
| –ClO1 | chlorosyl | =N2 | diazo |
| –ClO21 | chloryl | –N3 | azido |
| –ClO31 | perchloryl | –NCO5 | isocyanato |
| –F | fluoro | –NC | isocyano |
| –FO1 | fluorosyl | –NO1 | nitroso |
| –FO21 | fluoryl | –NO21 | nitro |
| –FO31 | perfluoryl | –S(O)-R2,6 | alkanesulfinyl |
| –I | iodo | –S(O)2-R2,6 | alkanesulfonyl |
| 1 And also chalcogen analogs, as thiochlorosyl, selenochloryl, dithiochloryl, and thionitroso. | |||
| 2 ‘R’ designates an ‘organic’ substituent group, as methoxy, pentyloxy, phenylperoxy, methanesulfonyl or methylsulfinyl, and benzenesulfonyl or phenylsulfonyl. | |||
| 3 Also included are chalcogen analogues, such as alkyl- or arylsulfanyl, alkyl- or arylselanyl, and alkyl- or aryltellanyl. | |||
| 4 Also included are chalcogen analogues, as methoxysulfanyl, methylsulfanyloxy, and methyldisulfanyl. | |||
| 5 Also included are chalcogen analogues, as isothiocyanato and isoselenocyanato. | |||
| 6 Includes selenium and tellurium analogues and all chalcogen analogues, as methanesulfinothioyl and benzeneselenotelluronyl. | |||
C6H5-NO
nitrosobenzene (PIN)
If characteristic groups other than those given in Table 5.1 are present, one (and only one) kind must be cited as suffix (the principal characteristic group) for classes other than radicals and ions.
When a compound contains more than one kind of characteristic group not given in Table 5.1, the principal characteristic group is the one that characterizes the class occurring earliest (i.e., nearest to the top) in the seniority order of classes (see P-41, P-42 and P-43, if necessary). All other characteristic groups are cited as prefixes.
If, and only if, the complete suffix (that is, the suffix plus its multiplying prefixes, if any) begins with a vowel, a terminal letter ‘e’ (if any) of the parent hydride name is elided. For example, ethanol (not ethaneol). Elision or retention of the terminal letter ‘e’ is independent of the presence of numerals between it and the following letter, for example, propan-2-ol (not propane-2-ol).
When a substituent is itself substituted (compound substituent, see P-29.4, P-35.3 and P-46), all the subsidiary substituents are named as prefixes. The substituent bearing the subsidiary substituent is regarded as a parent substituent (analogous to a parent hydride). The nomenclature of the whole substituent is subject to all the procedures adopted for compounds, with two exceptions, which are:
(a) that no characteristic group is expressed as a suffix (instead, a suffix such as ‘yl’, ‘ylidene’, etc., is used); andP-59.1.10 Numbering nomenclatural features(b) that the point of attachment of the substituent has the lowest permissible locant.
When the parent hydride (principal chain, ring, or ring system), principal group and substituents have been selected and named, the numbering of the complete compound is allocated using the rule of lowest locants. General rules for locants and numbering are described in P-14.4. They do apply each and every time a name is constructed, not only for substitutive and functional class nomenclature, but for all types of nomenclature.
The list of seniority of structural features that receive lowest possible locants has been refined by reallocating the placement of the ‘a’ prefixes for skeletal replacement (‘a’) nomenclature in chains and by giving a special status of detachable prefix to hydro/dehydro prefixes.
Insofar as the preceding rules leave a choice, the starting point and direction of numbering of a compound are chosen so as to give lowest locants to the structural features (if present) considered successively in the order given until a decision is reached:
(a) fixed numbering, as for naphthalene, bicyclo[2.2.2]octane, etc.;P-59.2 EXAMPLES ILLUSTRATING THE GENERAL METHODOLOGY(b) heteroatoms in heterocycles and in acyclic parent structures;
(c) indicated hydrogen [for unsubstituted compounds; a higher locant may be needed at another position to provide for a substituent suffix in accordance with structural feature (d)];
(d) principal group named as suffix;
(e) ‘added indicated hydrogen’ (consistent with the structure of the compound and in accordance with further substitution);
(f) saturation (‘hydro’/‘dehydro’ prefixes) or unsaturation (‘ene’/‘yne’ endings);
(g) substituents named as prefixes (low locants are allocated for substituents regardless of kind; then, if necessary, in the order of citation in the name).
P-59.2.1 Selection of parent hydridesP-59.2.1 Selection of parent compounds
P-59.2.2 Seniority of heteroatoms over suffixes
P-59.2.3 Seniority of principal characteristic groups over unsaturation
P-59.2.4 Seniority of ‘ene’ and ‘yne’ endings and hydro prefixes over detachable prefixes
P-59.2.5 Treatment of detachable prefixes
After the principal characteristic group has been chosen and named, the parent hydride or functional parent compound is chosen by one of the following methods. For details of numbering, see Chapter P-2 describing the numbering of the various parent hydrides and the general rule of lowest locants as formulated in P-14.3. For the arrangement of prefixes, see the general rule on alphanumerical order described in P-14.5.
P-59.2.1.1 If the compound is purely acyclic, the principal chain is chosen as parent hydride by the method described in P-44.
Example:
| Analysis | ||
| Principal group: | >C=O | one |
| Parent hydride: | CH3-CH2-CH2-CH2-CH2-CH3 | hexane |
| Functionalized parent hydride | CH3-CH2-CH2-CH2-CO-CH3 | hexan-2-one |
| Subtractive modification | CH3-CH2-CH=CH-CO-CH3 | hex-3-en-2-one |
| Substituents: | –Cl | chloro |
| –OH | hydroxy | |
| –CH3 | methyl | |
| Together with other rules, this analysis leads to the preferred IUPAC name: 3-chloro-6-hydroxy-5-methylhex-3-en-2-one (PIN) | ||
Explanation: The suffix ‘one’ receives the lowest possible locant, ‘2’, thus determining the direction of numbering of the chain. Two hexane chains are possible; the principal chain, in accord with the criteria for selecting the principal chain, is the one that is most substituted (3 substituents compared to 2). Unsaturation is denoted by the ending ‘ene’. The three substituent prefixes are arranged in alphanumerical order to complete the name.
P-59.2.1.2 If the principal group occurs only in a chain that carries a substituent, the compound is named as an acyclic compound and cyclic component is expressed by a substituent prefix.
Example:
| Analysis | ||
| Principal group: | (C)OOH | oic acid |
| Parent hydride: | CH3-CH2-CH2-CH2-CH2-CH3 | hexane |
| Functionalized parent hydride | CH3-CH2-CH2-CH2-CH2-COOH | hexanoic acid |
| Subtractive modification | CH3-CH2-CH=CH-CH2-COOH | hex-3-enoic acid |
| Substituents: | Cl– | chloro |
| C6H11– | cyclohexyl | |
| HO– | hydroxy | |
| CH3– | methyl | |
| Together with other rules, this analysis leads to the preferred IUPAC name: 3-chloro-5-cyclohexyl-6-hydroxy-5-methylhex-3-enoic acid (PIN) | ||
Explanation: The presence of a carboxylic acid group at the end of the chain determines the direction of its numbering. The ‘ene’ ending and the substituent prefixes, in alphanumerical order, are located on the chain in accord with its determined numbering.
P-59.2.1.3 If the principal group occurs in two or more carbon chains that are not attached to one another (that is, do not together form a continuous or branched chain but are separated by, for instance, a ring or a heteroatom), and when multiplicative nomenclature is not possible, then the chain carrying the largest number of the principal groups is chosen as the parent hydride for nomenclature; if the numbers of these groups in two or more chains are the same, choice is made by the principles for selection of the principal chain.
Example 1:
| Analysis | ||
| Principal group: | OH | diol |
| Parent hydride: | CH3-CH3 | ethane |
| Functionalized parent hydride | HO-CH2-CH2-OH | ethane-1,2-diol |
| Substituent: |  | |
| Substituent components: | OH | hydroxy |
| –CH2-CH2-CH3 | propyl | |
| –C6H5 | phenyl | |
| Substituent prefix: | 4-(3-hydroxypropyl)phenyl | |
| Together with other rules, this analysis leads to the preferred IUPAC name: 1-[4-(3-hydroxypropyl)phenyl]ethane-1,2-diol (PIN) | ||
Example 2: In the following example, the longest chain is chosen as parent hydride, in accord with the criteria for selecting the principal chain.
| Analysis | ||
| Principal group: | –OH | ol |
| Parent hydride: | CH3-CH2-CH3 | propane |
| Functionalized parent hydride | CH3-CH2-CH2-OH | propan-1-ol |
| Substituent: |  | |
| Substituent components: | –OH | hydroxy |
| –CH2-CH3 | ethyl | |
| –C6H5 | phenyl | |
| Substituent prefix: | 4-(2-hydroxyethyl)phenyl | |
| Together with other rules, this analysis leads to the preferred IUPAC name: 3-[4-(2-hydroxyethyl)phenyl]propan-1-ol (PIN) | ||
Example 3:
| Analysis | ||
| Principal group: | OH | ol |
| Parent hydride: | CH3-CH2-CH3 | propane |
| Functionalized parent hydride | 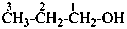 | propan-1-ol |
| Multiplicativ connecting group: | –C6H5– | 1,4-phenylene |
| Together with other rules, this analysis leads to the preferred IUPAC name: 3,3′-(1,4-phenylene)di(propan-1-ol) (PIN) | ||
Explanation: A multiplicative name is formed when identical parent structures are attached symmetrically to a central component (for a preferred IUPAC name the parent structures must be symmetrically substituted). The numbering of the multiplied parent structure, which includes the characteristic group, if any, is retained.
P-59.2.1.4 If the principal group occurs only in a single cyclic system, that cyclic system is chosen as parent hydride for nomenclature.
Example:
| Analysis | ||
| Principal group: | –OH | ol |
| Parent hydride: | C6H12 | cyclohexane |
| Functionalized parent hydride | C6H11-OH | cyclohexanol |
| Substituent: | –CH2-CH3 | ethyl |
| Together with other rules, this analysis leads to the preferred IUPAC name: 2-ethylcyclohexan-1-ol (PIN) | ||
P-59.2.1.5 If the principal group occurs in more than one cyclic system, the cyclic system chosen as parent hydride for nomenclature is in accordance with the criteria for choosing a senior ring or ring system.
| Analysis | ||
| Principal group: | –COOH | carboxylic acid |
| Parent hydride: |  | 9H-fluorene |
| Functionalized parent hydride |  | 9H-fluorene-2-carboxylic acid |
| Substituents: | –C6H5 | phenyl |
| –COOH | carboxy | |
| Together with other rules, this analysis leads to the preferred IUPAC name: 6-(4-carboxyphenyl)-9H-fluorene-2-carboxylic acid (PIN) | ||
P-59.2.1.6 If the principal group occurs both in a chain and in a cyclic system, the parent hydride for nomenclature is the portion in which the principal group occurs in the greater number; if the number of occurrences of the principal group is the same in two or more portions, the ring or ring system is chosen as parent hydride for nomenclature.
Example 1:
| Analysis | ||
| Principal group: | –OH | diol |
| Parent hydride: | CH3-CH2-CH2-CH2-CH2-CH3 | hexane |
| Functionalized parent hydride |  | hexane-1,6-diol |
| Substituent components: | –C6H11 | cyclohexyl |
| –OH | hydroxy | |
| Substituent prefix: | 4-hydroxycyclohexyl | |
| Together with other rules, this analysis leads to the preferred IUPAC name: 1-(4-hydroxycyclohexyl)hexane-1,6-diol (PIN) | ||
Example 2:
| Analysis | ||
| Principal group: | (C)O | dione |
| Parent hydride: |  | cyclopentane |
| Functionalized parent hydride | 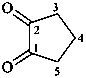 | cyclopentane-1,2-dione |
| Substituents: | =O | oxo |
| CH3-CH2-CH2-CH2– | butyl | |
| Substituent prefix: | 2-oxobutyl | |
| Together with other rules, this analysis leads to the preferred IUPAC name: 4-(2-oxobutyl)cyclopentane-1,2-dione (PIN) | ||
P-59.2.1.7 If the number of occurrences of the principal group is the same in two or more nomenclaturally significant parts of the compound, preferred IUPAC names are formed by choosing the ring or ring system as parent hydride for nomenclature.
Example:
| Analysis | ||
| Principal group: | –CHO | carbaldehyde |
| Parent hydride: |  | cyclohexane |
| Functionalized parent hydride |  | cyclohexanecarbaldehyde |
| Substituent: | –CH2-[CH2]5-CHO | 7-oxoheptyl |
| Together with other rules, this analysis leads to the preferred IUPAC name: 3-(7-oxoheptyl)cyclohexane-1-carbaldehyde (PIN) | ||
In general nomenclature, a chain may be chosen as the parent hydride, depending on the importance given to a specific portion (Rule P-44.1.2.2), leading to the name: 7-(3-formylcyclohexyl)heptanal
P-59.2.1.8 When a substituent is itself substituted, all the subsidiary substituents are named as prefixes. The substituent bearing the subsidiary substituents is regarded as a ‘parent substituent’ (analogous to a parent compound). The nomenclature of the whole substituent is subject to all the procedures adopted for compounds (for instance, choice of principal chain), with two exceptions, namely: (a) that no suffix is used, and (b) that the point of attachment of the substituent bears the lowest permissible locant number depending on the nomenclature of the substituent group, alkyl or alkanyl.
Example:
| Analysis | ||
| Principal group: | –COOH | carboxylic acid |
| Parent hydride: |  | cyclohexane |
| Functionalized parent hydride |  | cyclohexanecarboxylic acid |
| Primary substituents: | –Cl | chloro |
| –CH2-CH2-CH2-CH2-CH2-CH3 | hexyl | |
| Secondary substituents: | –Cl | chloro |
| =O | oxo | |
| –CH2-OH | hydroxymethyl | |
| Substituted substituents: | 4-chloro-2-(hydroxymethyl)-5-oxohexyl | |
| Together with other rules, this analysis leads to the preferred IUPAC name: 4,5-dichloro-2-[4-chloro-2-(hydroxymethyl)-5-oxohexyl]cyclohexane-1-carboxylic acid (PIN) | ||
P-59.2.2 Seniority of heteroatoms over suffixes
Heterocyclic compounds and chains modified by skeletal replacement (‘a’) nomenclature are treated similarly. They are considered as parent compounds with a fixed numbering. As a consequence, heteroatoms have seniority for low locants and suffixes are assigned the next possible lowest locants.
Fixed numbering for heteroacyclic parent structures named by skeletal replacement (‘a’) nomenclature is a major change to Rule C-0.6 (ref. 1) in which principal characteristic groups and free valence were preferred over heteroatoms for low locants.
P-59.2.2.1 For chains the replacement operation is applied to the hydrocarbon parent hydride to create a new parent hydride with a fixed numbering. Suffixes receive the lowest possible locants in accordance with the resulting numbering.
Example:
| Analysis | ||
| Principal characteristic group: | –(C)OOH | oic acid |
| Hydrocarbon parent hydride: | CH3-[CH2]13-CH3 | pentadecane |
| Skeletal replacement (‘a’) prefix | –O– | oxa |
| Heteroacyclic parent hydride: | ||
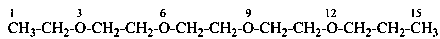 | ||
| 3,6,9,12-tetraoxapentadecane | ||
| Functionalized heteroacyclic parent hydride: | ||
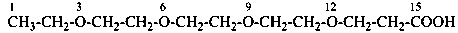 | ||
| Together with other rules, this analysis leads to the preferred IUPAC name: 3,6,9,12-tetraoxapentadecan-15-oic acid (PIN) | ||
P-59.2.2.2 Heterocyclic compounds having retained or systematic names are considered as parent hydrides. Thus, suffixes are added and assigned lowest possible locants in accordance with the fixed numbering of the heterocyclic ring or ring system. Added indicated hydrogen atoms, if needed, are assigned next lowest possible locants.
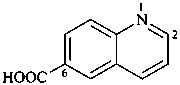
quinolin-6(2H)-one (PIN)
| Analysis | ||
| Principal characteristic group: | –COOH | carboxylic acid |
| Hydrocarbon parent hydride: | 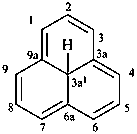 | 3a1H-phenalene |
| [Note the new system of numbering for internal atoms in fused systems, 3a1 in place of 9b; see P-25.3.3.3.] | ||
| Skeletal replacement (‘a’) prefix | –N< | aza |
| Heterocyclic parent hydride: | 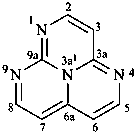 | 1,3a1,4,9-tetraazaphenalene |
| Functionalized heterocyclic parent hydride: | 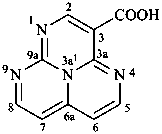 | |
| Together with other rules, this analysis leads to the preferred IUPAC name: 1,3a1,4,9-tetraazaphenalene-3-carboxylic acid (PIN) | ||
P-59.2.3 Seniority of suffixes over unsaturation
P-59.2.3.1 After suffixes, if there is a choice, low locants are assigned to ‘ene’ and ‘yne’ endings, and then to detachable prefixes, if applicable.
Example:
| Analysis | ||
| Principal characteristic group: | =O | one |
| Parent hydride: | CH3-[CH2]5-CH3 | heptane |
| Functionalized parent hydride | CH3-[CH2]2-CO-[CH2]2-CH3 | heptan-4-one |
| Substituent prefix | –F | fluoro |
| Together with other rules, this analysis leads to the preferred IUPAC name: 7,7,7-trifluorohept-1-en-4-one (PIN) | ||
P-59.2.3.2 Hydro and dehydro prefixes are used to express a change in the degree of hydrogenation of the parent hydride. In these recommendations, these prefixes are considered detachable but only in the context of numbering; they are not included among the detachable substituent prefixes. In names, they are cited immediately before the name of the parent compound, after those of detachable substituent prefixes arranged in alphanumerical order.
In these recommendations, the prefixes ‘hydro’ and ‘dehydro’ are detachable, but are not included in the category of alphabetized detachable prefixes (see P-14.4; see also P-15.1.5.2, P-31.2, P-58.2), which is a change from recommendations in earlier editions (ref. 1, 2). When along with the endings ‘ene’ and ‘yne’ they are used to modify parent hydrides, they are regulated by the principle of lowest locants, in accord with the numbering of the parent hydride and after priority has been given to indicated hydrogen, ‘added indicated hydrogen’, and suffixes, when present, as specified in the general rules for numbering (P-14.4).
Example:
| Analysis | ||
| Principal characteristic group: | –COOH | carboxylic acid |
| Parent hydride: |  | azulene |
| Functionalized parent hydride |  | azulene-2-carboxylic acid |
| Saturation prefix | –H | dihydro |
| Substituent prefix | –Br | bromo |
| Together with other rules, this analysis leads to the preferred IUPAC name: 7-bromo-5,6-dihydroazulene-2-carboxylic acid (PIN) | ||
P-59.2.3.3 Mancude ketones, imines, and other characteristic groups as well as free valences, such as ‘-ylidene’ are named by the ‘added indicated hydrogen’ method. If there is a choice, indicated hydrogen atoms have priority for low locants, then suffixes, ‘added indicated hydrogen’ atoms, and finally hydro prefixes, in that order.
Example 1:
| Analysis | ||
| Principal characteristic group: | =O | one |
| Parent hydride: |  | naphthalene |
| Functionalized parent hydride |  | naphthalen-2(4aH)-one |
| Saturation prefix | 4 –H | tetrahydro |
| Together with other rules, this analysis leads to the preferred IUPAC name: 5,6,7,8-tetrahydronaphthalen-2(4aH)-one (PIN) | ||
Example 2:
| Analysis | ||
| Free valences: | R= | ylidene |
| Parent hydride: |  | quinoline |
| Parent hydride with free valences |  | quinolin-2(1H)-ylidene |
| Together with other rules, this analysis leads to the preferred IUPAC name: 3,4-dihydroquinolin-2(1H)-ylidene (preferred prefix) | ||
Example 3:
| Analysis: | ||
| Principal characteristic group: | =O | dione |
| Parent hydride: |  | 1H-indene |
| Functionalised parent hydride: |  | 1H-indene-1,4(2H)-dione |
| Saturation prefix: | dihydro | |
| Together with other rules, this analysis leads to the preferred IUPAC name: 3,3a-dihydro-1H-indene-1,4(2H)-dione | ||
| Analysis | ||
| Principal characteristic group: | 2 =O | dione |
| Parent hydride: |  | 1H-cyclopenta[a]naphthalene |
| Functionalized parent hydride |  | 1H-cyclopenta[a]naphthalene-3,5(2H,4H)-dione |
| Explanation: See P-58.2.2 | ||
| Saturation prefix | 2 –H | dihydro |
| Together with other rules, this analysis leads to the preferred IUPAC name: 3a,9b-dihydro-1H-cyclopenta[a]naphthalene-3,5(2H,4H)-dione (PIN) | ||
Example 5:
| Analysis | ||
| Principal characteristic group: | R– | yl |
| Parent hydride: |  | 1H-isoindole |
| Parent hydride with free valence | 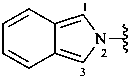 | 2H-isoindol-2-yl |
| Saturation prefix | 2 –H | dihydro |
| Substituent: | =O | dioxo |
| Together with other rules, this analysis leads to the preferred IUPAC name: 1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl (preferred prefix) | ||
P-59.2.4 Seniority of ‘ene’ and ‘yne’ endings and hydro prefixes over detachable prefixes.
If there is a choice, low locants are assigned first to ‘ene’ and ‘yne’ endings and ‘hydro/dehydro’ prefixes, then to detachable alphabetized prefixes.
Examples:
 | compare with |  |
| 1,3,3-trimethylcyclohex-1-ene (PIN) | 1,1,3-trimethylcyclohexane (PIN) | |
 | compare with | 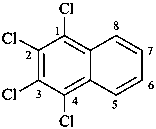 |
| 5,6,7,8-tetrachloro-1,2,3,4-hydronaphthalene (PIN) | 1,2,3,4-tetrachloronaphthalene (PIN) |
P-59.2.5 Treatment of detachable prefixes
If there is a choice, low locants are assigned to detachable prefixes considered together, and, if there is a further choice, in alphanumerical order.
Examples:
1-bromo-3-chloroazulen-6-ol (PIN)
(2R)-2-bromo-1-chloro-1,1-difluoro-2-iodoethane (PIN)
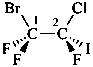
(2S)-1-bromo-2-chloro-1,1,2-trifluoro-2-iodoethane (PIN)
Return to:
Blue Book home page.