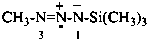
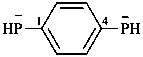
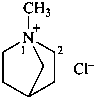
3-methyl-1-(trimethylsilyl)triaz-2-en-2-ium-1-id-2-yl (PIN)
Division VIII Chemical Nomenclature and Structure Representation Division
P-70 IntroductionP-70 INTRODUCTION
P-71 Radicals
P-72 Anions
P-73 Cations
P-74 Zwitterions
P-75 Radical ions
P-76 Delocalized radicals and ions
P-77 Salts
P-70.1 General MethodologyP-70.1 GENERAL METHODOLOGY.
P-70.2 Seniority of radicals and ions
P-70.3 Name formation
P-70.4 General rules for the selection of preferred names
The nomenclature for radicals, ions and related species is described in this Chapter. Its rules are based on the same principles as those of organic compounds defined in the Chapters P-1 to P-6. The nomenclature was revised in 1993 (ref. 3). For definitions, symbols and conventions, see ref. 14; see also ref. 28. In the 1979 recommendations (ref. 1), radicals were called ‘free radicals’ to distinguish them from substituent prefixes which were also called radicals. That distinction was dropped in the 1993 publications (refs. 2, 3).
P-70.2 SENIORITY OF RADICALS AND IONS
As classes, radicals and ions are senior to acids and other classes in the following order:
(1) radicals;P-70.3 NAME FORMATION
(2) anions;
(3) cations.
Substitutive names and functional class names denote radicals and ions and related compounds. Parent hydrides and parent compounds are selected and modified by use of specific suffixes (called cumulative suffixes) and prefixes; traditional endings are used to describe anions derived from acids and related compounds (see P-72.2.2.2). The nomenclature of di- and trivalent radicals does not indicate nor imply an electronic structure or spin multiplicity.
P-70.3.1 Suffixes, prefixes, and endings for radicals and ions in substitutive nomenclature are listed in Table 7.1. They are also described in Table 3.4.
| Operation | Suffix or Ending | Prefix | ||
| Radicals formed by | ||||
| loss of H• | yl | ylo | ||
| loss of 2 H• | ||||
| from one atom | ylidene | |||
| from different atoms | diyl | |||
| loss of 3 H• | ||||
| from one atom | ylidyne | |||
| from different atoms | triyl or ylylidene | |||
| addition of H• | hydryl | |||
| Anions formed by | ||||
| loss of H+ | ide | |||
| ate, ite (endings) | ||||
| addition of H– | uide | |||
| addition of an electron | elide 1 | |||
| Cations formed by | ||||
| loss of H– | ylium | |||
| addition of H+ | ium | |||
| loss of an electron | elium 1 | |||
| 1 The suffixes ‘elide’ and ‘elium’ are recommended to denote modification of a parent hydride by the addition or the subtraction of one electron, respectively. | ||||
P-70.3.3 In names, suffixes and endings are cited in a specific order as described below.
P-70.3.3.1 When two or more cumulative suffixes are present in a name, the order of citation is the reverse of the order of seniority for radicals and ions as given in P-70.2, i.e.,‘ium’, ‘ylium’, ‘ide’, ‘uide’, ‘yl’, ‘ylidene’, ‘ylidyne’.
Example:
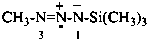
P-70.3.3.2.1 A cumulative suffix may be added to a functional suffix to form a defined compound suffix (see P-71.3.2).
Examples:
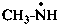
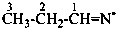
propan-1-iminyl (PIN)
propylideneazanyl
Example:
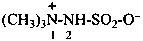
The concept of preferred IUPAC names as applied to radicals and ions is based on the following principles.
(1) substitutive nomenclature based on carbane and heterane nomenclatures and a set of suffixes and prefixes designed to express the formal operations needed to generate radicals and ions systematically are used to generate preferred IUPAC names; accordingly, the preferred IUPAC name for a radical may not be the same as the preferred prefix.
(2) some names are retained as preferred IUPAC names, notably contracted names such as methoxide, ethoxide, etc., and methoxyl, ethoxyl, etc. related to the substitutive prefixes derived from alcohols and related hydroxy compounds.
(3) some names are retained only for use in general nomenclature, for example the ‘onium cations’ such as ammonium and sulfonium, carbene, CH22•; amide, NH2–; and CH3-C(O)– , acetyl anion.
(4) functional class names using class names such as cation, anion, etc. can be used in general nomenclature, but systematically constructed names or retained names are preferred IUPAC names, for example, ‘methylium’ not ‘methyl cation’, for CH3+; ‘acetylium’ not ‘acetyl cation’ for CH3-C(O)+; ‘ethanide’ not ‘ethyl anion’ for CH3-CH2–; and ‘methaniumyl’ not ‘methyl radical cation’, for CH4•+.
P-71.1 General methodologyP-71.1 GENERAL METHODOLOGY
P-71.2 Radicals derived from parent hydrides
P-71.3 Radical centers on characteristic groups
P-71.4 Assemblies of parent radicals
P-71.5 Prefixes denoting radicals
P-71.6 Order of citation and seniority of suffixes ‘yl’, ‘ylidene’, and ‘ylidyne’
P-71.7 Choice of parent radical
Radicals are named by modifying a parent hydride name to signal the subtraction or addition of one or more hydrogen atoms, H•. The modification to signal the addition of a single hydrogen atom is recommended for the first time. These two operations are expressed by suffixes.
The suffixes ‘yl’ (–H•), ‘ylidene’ (–2H•), ‘ylidyne’ (–3H•) denote the removal of hydrogen atoms, a subtractive operation.
The suffix ‘hydryl’ denotes the additive operation, i.e., the addition of a single hydrogen atom.
The prefix ‘ylo’ is used to indicate the removal of ‘H•’ from a substituent group, a subtractive operation.
P-71.2 RADICALS DERIVED FROM PARENT HYDRIDES
P-71.2.1 Monovalent radicals.
P-71.2.1.1 A radical formally derived by the removal of one hydrogen atom from a mononuclear parent hydride of an element of Group 14, from a terminal atom of an unbranched acyclic hydrocarbon, or from any position of a monocyclic saturated hydrocarbon ring is named by replacing the ‘ane’ ending of the systematic name of the parent hydride by ‘yl’.
Examples:
•CH2-CH2-CH3
propyl (PIN)
•GeH3
germyl (preselected name)
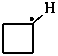
cyclobutyl (PIN)
Examples:
H2N•
azanyl (preselected name)
aminyl (traditional name: amino)
H2B•
boranyl (preselected name)
(not boryl)
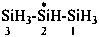
trisilan-2-yl (preselected name)
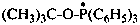
tert-butoxytri(phenyl)-λ5-phosphanyl (PIN)
[(2-methylpropan-2-yl)oxy]tri(phenyl)-λ5-phosphanyl
(1,1-dimethylethoxy)tri(phenyl)-λ5-phosphanyl
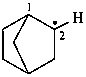
bicyclo[2.2.1]heptan-2-yl (PIN)
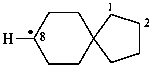
spiro[4.5]decan-8-yl (PIN)
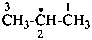
propan-2-yl (PIN)
1-methylethyl
isopropyl
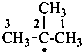
2-methylpropan-2-yl (PIN)
1,1-dimethylethyl
tert-butyl [see P-70.4 (1)]
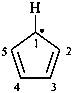
cyclopenta-2,4-dien-1-yl (PIN)
cyclopentadienyl (see P-76)
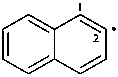
naphthalen-2-yl (PIN)
Example:
The names of divalent and trivalent radicals are formed substitutively using the suffixes ‘ylidene’ and ‘ylidyne’ in two ways:
(1) replacing the ending ‘ane’ of a mononuclear parent hydride of an element of Group 14, or from a terminal atom of an unbranched acyclic hydrocarbon, or from any position of a monocyclic saturated hydrocarbon ring by the appropriate suffix (corresponds to P-71.2.1.1)These systematic names are preferred to retained names which may be used in general nomenclature.(2) adding the appropriate suffix to the name of a parent hydride, other than those described by P-71.2.1.1, at any position eliding the final letter ‘e’ of the name of the parent hydride, if any (corresponds to P-71.2.1.2).
P-71.2.2.1 Specific method and retained names
A radical formally derived by the removal of two hydrogen atom from one skeletal atom of a mononuclear parent hydride of an element of Group 14, or from one terminal skeletal atom of an unbranched acyclic hydrocarbon, or from one skeletal atom of a monocyclic saturated hydrocarbon ring is named by replacing the ‘ane’ ending of the systematic name of the parent hydride by the suffix ‘-ylidene’ or ‘-diyl’. The suffix ‘-ylidyne’ or ‘-triyl’ is used to name radicals formally derived by the removal of three hydrogen atoms from a mononuclear parent hydride of an element of Group 14 or from a terminal atom of an unbranched acyclic hydrocarbon.
Systematic names are the preferred IUPAC names. The retained names carbene or methylene, nitrene or aminylene and carbyne, can be used in general nomenclature, with full substitution. The use of the systematic or retained names does not imply a specific electronic configuration. If needed, such a distinction would be made by using a separate word such as singlet or triplet, or a descriptive phrase. The disposition of the two unpaired electrons in the structures is equivalent to that given in the Red Book as CH22• (see ref. 12, IR-6.4.7).
Examples:
H2Si2•
silylidene (preselected name)
silanediyl
(not silylene)
HC3•
methylidyne (PIN)
methanetriyl
carbyne
(C6H5)2C2•
diphenylmethylidene (PIN)
diphenylmethanediyl
diphenylcarbene
diphenylmethylene
C6H5-CH2-SiH2•
benzylsilylidene (PIN)
benzylsilanediyl
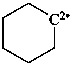
cyclohexylidene (PIN)
cyclohexane-1,1-diyl
CH3C3•
ethylidyne (PIN)
ethane-1,1,1-triyl
(not methylcarbyne)
With the exception of the radicals named in P-71.2.2.1, the names of divalent and trivalent radicals derived by the removal of two or three hydrogen atoms from one position of a parent hydride are formed by adding the suffixes ‘-ylidene’ or ‘-diyl’ and ‘-ylidyne’ or ‘-triyl’, respectively, to the name of the parent hydride, with elision of the final letter ‘e’, if present. The name azanylidene is the preselected name for HN2•; nitrene or aminylidene are retained names for use in general nomenclature.
Examples:
H2P3•
λ5-phosphanylidyne (preselected name)
λ5-phosphanetriyl
phosphoranylidyne
phosphoranetriyl
H2N-N2•
hydrazinylidene (preselected name)
diazanylidene
hydrazine-1,1-diyl
diazane-1,1-diyl
(traditional name: hydrazono)
(not aminonitrene)
H2P-P2•
diphosphanylidene (preselected name)
diphosphane-1,1-diyl
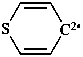
4H-thiopyran-4-ylidene (PIN)
4H-thiopyran-4,4-diyl
Polyradicals containing two or more radicals centers, formally derived by the removal of two or more hydrogen atoms from each of two or more different skeletal atoms of a parent hydride, are named by adding to the name of the parent hydride combinations of the suffix ‘yl’ for a monovalent radical center, ‘ylidene’ for a divalent radical center, and ‘ylidyne’ for a trivalent radical center, together with the appropriate numerical prefixes indicating the number of each kind of radical center. The final letter ‘e’ of the name of the parent hydride, if present, is elided when followed by ‘y’. All substituents, including characteristic groups, when present, are cited as prefixes. Preferred IUPAC names result from the application of this rule.
Examples:
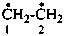
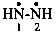
hydrazine-1,2-diyl (preselected name)
diazane-1,2-diyl
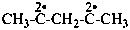
pentane-2,4-diylidene (PIN)
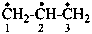
propane-1,2,3-triyl (PIN)
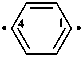
benzene-1,4-diyl (PIN)
{traditional names: p-phenylene;
1,4-phenylene [see P-70.4 (1)]}
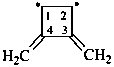
3,4-dimethylidenecyclobutane-1,2-diyl (PIN)
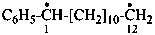
1-phenyldodecane-1,12-diyl (PIN)
(1) by citing the locant of the nonterminal position of the chainMethod (1) generates preferred IUPAC names. The principal chain is chosen, if necessary, by the method indicated in Section P-46 for substituent groups.(2) by substituting a parent radical that has the free valence(s) at the end of a chain.
Example:
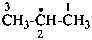
Divalent and trivalent radical centers in a parent hydride formally derived by the removal of two or three hydrogen atoms from the same skeletal atom in its standard valence state may be described by the λ-convention (see P-14.1). Locants for the radical centers are followed by the symbol λn, where ‘n’ is the bonding number of the skeletal atom (see P-14.1). This method is only for general nomenclature.
Examples:
FC3•
fluoro-λ1-methane
fluoromethylidyne (PIN)
fluoromethanetriyl
C6H5-N2•
phenyl-λ1-azane
benzenaminylidene (PIN)
phenylazanediyl
A radical center at a position in a mancude parent hydride where there is an insufficient number of hydrogen atoms to apply directly the recommendations for the use of ‘yl’ or ‘ylidene’ given in P-71.2.1 and P-71.2.2 is derived formally from a dihydro derivative of the cyclic parent hydride. Such a radical can also be described by applying the principle of ‘added indicated hydrogen’ (see P-14.7 and P-58.2). In this method the ‘hydro’ derivative is described by specifying the hydrogen atom of a dihydro pair that remains after the radical center is created, by citing in italic capital H and the locant of the skeletal atom to which the hydrogen atom resides, both enclosed in a set of parentheses and inserted into the name of the corresponding parent hydride immediately after the locant for the radical center.
Names formed by the ‘added indicated hydrogen’ method are preferred to names using ‘hydro’ prefixes (see P-58.2.5).
Examples:
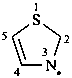
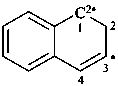
naphthalen-3-yl-1(2H)-ylidene (PIN)
1,2-dihydronaphthalen-3-yl-1-ylidene
(nondetachable hydro prefixes; see P-58.2.5)
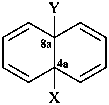
X = •; Y = H
naphthalen-4a(8aH)-yl (PIN)
4a,8a-dihydronaphthalen-4a-yl
(nondetachable hydro prefixes, see P-58.2.5)
X = •; Y = •
naphthalene-4a,8a-diyl (PIN)
4a,8a-dihydronaphthalene-4a,8a-diyl
(nondetachable hydro prefixes, see P-58.2.5)
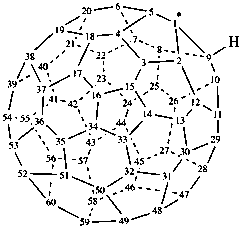
(C60-Ih)[5,6]fulleren-1(9H)-yl (PIN)
1,9-dihydro(C60-Ih)[5,6]fulleren-1-yl
(nondetachable hydro prefixes, see P-58.2.5)
P-71.3.1 Acyl radicals
Acyl radicals, i.e., radicals with at least one chalcogen or nitrogen atom attached to a radical center by a (formal) double bond, which may be considered to be formally derived by the removal of a hydroxy group from acid characteristic groups, are named by replacing the ‘ic acid’ or ‘carboxylic acid’ ending of the name of the acid with ‘oyl’ or ‘yl’, or ‘carbonyl’, according to the method for forming names of acyl groups (see P-65.1.7). Substituent groups denoted by prefixes such as ‘oxo’, ‘thioxo’, ‘sulfanylidene’, etc., may be used in general nomenclature.
Compound acyl radicals formed from acyclic parent hydrocarbons and substituent prefixes such as ‘oxo’, ‘thioxo’, ‘sulfanylidene’, and ‘imino’ can be used in general nomenclature; they are used in CAS index nomenclature.
Examples:
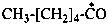
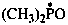
dimethylphosphinoyl (PIN)
dimethyl(oxo)-λ5-phosphanyl
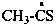
ethanethioyl (PIN)
1-sulfanylideneethyl
1-thioxoethyl
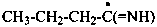
butanimidoyl (PIN)
1-iminobutyl
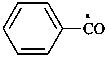
benzoyl (PIN)
benzenecarbonyl
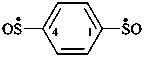
benzene-1,4-disulfinyl (PIN) (1,4-phenylene)bis(oxo-λ4-sulfanyl)
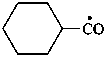
cyclohexanecarbonyl (PIN)
cyclohexyl(oxo)methyl
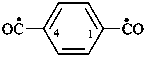
benzene-1,4-dicarbonyl (PIN)
terephthaloyl
(1,4-phenylene)bis(oxomethyl)
| –NH2 | amine (preselected suffix) | 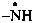 | aminyl (preselected suffix) |
| –N2• | aminylidene (preselected suffix) | ||
| =NH | imine (preselected suffix) | =N• | iminyl (preselected suffix) |
| –(C)O-NH2 | amide (preferred suffix) | 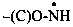 | amidyl (preferred suffix) |
| –(C)O-N2• | amidylidene (preferred suffix) | ||
| –CO-NH2 | carboxamide (preferred suffix) | 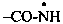 | carboxamidyl (preferred suffix) |
| –CO-N2• | carboxamidylidene (preferred suffix) |
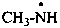
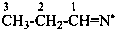
propan-1-iminyl (PIN)
propylideneazanyl
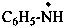
benzenaminyl (PIN)
phenylaminyl
phenylazanyl
(traditionally: phenylamino)
(not anilino)
(CH3)3P=N•
trimethyl-λ5-phosphaniminyl (PIN)
trimethylphosphane imidyl
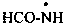
formamidyl (PIN)
formylazanyl
formylaminyl
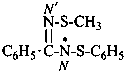
N′-(methylsulfanyl)-N-(phenylsulfanyl)benzenecarboximidamidyl (PIN)
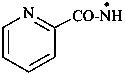
pyridine-2-carboxamidyl (PIN)
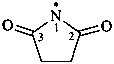
2,5-dioxopyrrolidin-1-yl (PIN)
succinimidyl
C6H5-N2•
benzenaminylidene (PIN)
phenylnitrene
phenylaminylene
CH3-CO-N2•
acetamidylidene (PIN)
acetylnitrene
acetylaminylene
Polyradicals with radical centres identically derived but located on two or more amine, imine, or amide characteristic groups are named in two ways:
(1) by using suffixes (see P-71.3.2) denoting the removal of one hydrogen atom from each characteristic group and the multiplying prefixes ‘bis-’, ‘tris-’, etc.;In order to avoid any confusion, the name ‘aminyl’ is reserved for denoting the suffix in substitutive nomenclature; the parent radical ‘azanyl’ (not ‘aminyl’) is used in multiplicative nomenclature. Method (1) leads to preferred IUPAC names when a suffix described in P-71.3.2 is available.(2) by multiplicative nomenclature based on the parent radicals ‘azanyl’ and ‘azanylidene’.
Examples:
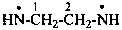
•N=C=N•
(1) methanebis(iminyl) (PIN)
(2) methanediylidenebis(azanyl)
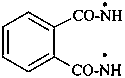
(1) benzene-1,2-bis(carboxamidyl) (PIN)
(2) (benzene-1,2-dicarbonyl)bis(azanyl)
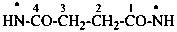
(1) butanebis(amidyl) (PIN)
(2) butanedioylbis(azanyl)
2•N-CO-[CH2]4-CO-N2•
(1) hexanebis(amidylidene) (PIN)
(2) hexanedioylbis(azanylidene)
hexanedioylbis(aminylidene)
hexanedioylbis(nitrene)
(1) additively, using the term ‘oxyl’ or ‘peroxyl’ derived from the terms ‘oxy’ or ‘peroxy’ (not dioxy);The names methoxyl, ethoxyl, propoxyl, butoxyl, tert-butoxyl, phenoxyl, and aminoxyl, which may be considered as contractions of the systematically formed names, such as methanyloxyl or methyloxyl, are retained and are preferred IUPAC names (see P-63.2.2.2 for names such as methoxy, ethoxy, etc.).(2) by substituting the parent radicals ‘oxidanyl’ (preselected name), for HO•, or ‘dioxidanyl’ (preselected name), for HOO•, by the appropriate substituent groups.
Method (1) generates preferred IUPAC names.
Examples:
ClCH2-CO-O•
(1) (chloroacetyl)oxyl (PIN)
chloroacetoxyl
(2) (chloroacetyl)oxidanyl
H2N-O•
aminoxyl (preselected name;
a contraction of aminooxyl)
(ClCH2)2N-O•
(1) bis(chloromethyl)aminoxyl (PIN)
(2) [bis(chloromethyl)amino]oxidanyl
CH3-[CH2]4-CO-O-O•
(1) hexanoylperoxyl (PIN)
(2) hexanoyldioxidanyl
CH3-[CH2]3-O•
(1) butoxyl (PIN)
(2) butyloxidanyl
CH3-[CH2]2CO-O•
(1) butanoyloxyl (PIN)
(2) butanoyloxidanyl
Examples:
CH3-Se•
methylselanyl (PIN)
CH3-C(CH3)2-SS•
tert-butyldisulfanyl (PIN)
(2-methylpropan-2-yl)disulfanyl
ClCH2-CS-S•
(chloroethanethioyl)sulfanyl (PIN)
Polyradicals with radical centers identically derived from the same parent hydride or the same characteristic group (except for polyacyl or polyamide radicals described in P-71.3.1 and P-71.3.3, respectively) but located in different parts of the structure are named, if possible, according to the principles for nomenclature of assemblies of identical units linked by multivalent substituents (see P-15.3).
Examples:
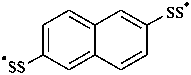
(naphthalene-2,6-diyl)bis(disulfanyl) (PIN)
•O-C(CH3)2-CH2-C(CH3)2-O•
(1) (2,4-dimethylpentane-2,4-diyl)bis(oxyl) (PIN)
(1,1,3,3-tetramethylpropane-1,3-diyl)bis(oxyl)
(2) (2,4-dimethylpentane-2,4-diyl)bis(oxidanyl)
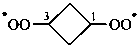
(1) (cyclobutane-1,3-diyl)bis(peroxyl) (PIN)
(2) (cyclobutane-1,3-diyl)bis(dioxidanyl)
The presence of a radical center in a substituent that is to be cited as a prefix is expressed in two ways:
(1) by using the prefix ‘ylo’ that indicates the subtraction of a hydrogen atom from a substituent group, for example ‘–ylomethyl’ for –CH2•;
(2) by concatenation of prefixes, for example ‘oxylcarbonyl’ for –CO-O•.
This prefix is a nondetachable prefix, attached to the parent substituent prefix, which is formed by usual methods. The presence of two or more radical centers in a substituent cited as a prefix or the removal of two or more hydrogen atoms from a substituent cited as prefix is indicated by the appropriate multiplying prefix, ‘di’, ‘tri’, etc.
Examples:
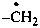
–O•
ylooxidanyl (preselected prefix)
ylooxy
(not ylohydroxy)
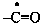
yloformyl (preferred prefix)
–CO-O•
oxylcarbonyl (preferred prefix)
(ylooxidanyl)formyl
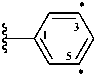
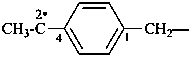
3,5-diylophenyl (preferred prefix)
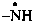
yloamino (preselected prefix)
yloazanyl
[4-(1,1-diyloethyl)phenyl]methyl (preferred prefix)
The suffixes ‘yl’, ‘ylidene’, and ‘ylidyne’ are cited in that order in a name, if applicable; lowest locants are assigned to radicals as a set, then in the order ‘yl’, ‘ylidene’ and ‘ylidyne’. The order of citation is identical to that used for naming substituent groups (see P-29.3.2.2).
Example:
When a choice of a parent radical is necessary, the following criteria are applied, in the order given, until a decision is reached.
(a) Parent with the maximum number of radical centers of any kind in a single parent structure:
Example:
Example:
The seniority order for radicals is now the order of seniority of classes rather than the order of skeletal replacement (‘a’) prefixes as used in RC-81.3.3.2, ref. 3.
Example:
(1) maximum number of radical centers according to the order of suffixes (see P-33).P-72 ANIONSExample:
(2) rings are senior to chains 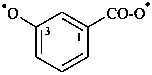
[3-(ylooxidanyl)benzoyl]oxyl (PIN)
[3-(ylooxidanyl)benzoyl]oxidanyl
{not 3-[(ylooxidanyl)carbonyl]phenoxyl;
ylooxidanyl is senior to phenoxyl}
Example:
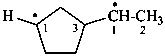
3-(1-yloethyl)cyclopentyl (PIN)
[not 1-(3-ylocyclopentyl)ethyl;
cyclopentyl is senior to ethyl]
P-72.1 General methodologyP-72.1 GENERAL METHODOLOGY
P-72.2 Anions formed by removal of hydrons
P-72.3 Anions formed by addition of hydride ions
P-72.4 Skeletal replacement nomenclature
P-72.5 Multiple anionic centers
P-72.6 Anionic centers in both parent compounds and substituent groups
P-72.7 Choice of an anionic parent structure
P-72.8 The suffixes ‘ide’ and ‘uide’ and the λ-convention
Anions are named in two ways:
(1) by using suffixes and endings;Method (1) leads to preferred IUPAC names. Some names and some contracted names are retained as preferred IUPAC names and for use in general nomenclature.(2) by functional class nomenclature.
The following suffixes are used:
‘ide’ (preferred suffix; corresponding to removal of a hydron, H+),The endings ‘ate’ and ‘ite’ are used to indicate removal of a hydron from the –OH group of acids and hydroxy compounds.‘uide’ (preferred suffix; corresponding to the addition of a hydride ion, H–),
‘elide’ (preferred suffix; corresponding to the addition of an electron)
Functional class nomenclature is based on the class name ‘anion’ in association with the name of the corresponding radical (not necessarily the name of the corresponding substituent group).
P-72.2 ANIONS FORMED BY REMOVAL OF HYDRONS
P-72.2.1 Functional class nomenclatureP-72.2.1 Functional class nomenclature
P-72.2.2 Systematic nomenclature
Functional class nomenclature can be used, in general nomenclature, to describe anionic compounds. An anion that can be considered as derived formally by adding an electron to a radical may also be named by adding the class name ‘anion’ as a separate word to the name of the substituent group. The names are formed by using the names of corresponding radicals (not necessarily the name of substitutent groups) and the class name ‘anion’ as a separate word. The multiplying prefixes ‘di’, ‘tri’, etc., are added to the class name to denote multiple anions. This type of nomenclature is limited to anions having anionic centers in the same structure. Systematic names (see P-72.2.2) are preferred IUPAC names.
Examples:
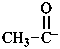
acetyl anion
1-oxoethan-1-ide (PIN)
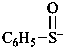
benzenesulfinyl anion
oxo(phenyl)-λ4-sulfanide (PIN)
CH3-NH–
methanaminyl anion
methanaminide (PIN)
(C6H5)2C2–
diphenylmethylidene dianion
diphenylmethanediide (PIN)
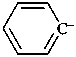
phenyl anion
benzenide (PIN)
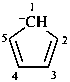
cyclopenta-2,4-dien-1-yl anion
cyclopenta-2,4-dien-1-ide (PIN)
cyclopentadienide (see P-76)
P-72.2.2.1 Anions derived from parent hydrides and their derivatives
An anion derived formally by the removal of one or more hydrons from any position of a neutral parent hydride is preferably named by using the suffix ‘-ide’, with elision of the final letter ‘e’ of the parent hydride, if any. Numerical prefixes ‘di’, ‘tri’, etc. are used to denote multiplicity; locants identify positions of the negative charges.
The name ‘acetylide’, for –C≡C–, is retained for general use only.
Examples:
(C6H5)2C2–
diphenylmethanediide (PIN)
(CH3)2P–
dimethylphosphanide (PIN)
dimethylphosphinide
HC≡Si–
methylidynesilanide (PIN)
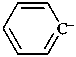
benzenide (PIN)
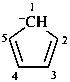
cyclopenta-2,4-dien-1-ide (PIN)
cyclopentadienide (see P-76)
–C≡C–
ethynediide (PIN)
acetylide
An anionic center at a position in a mancude parent hydride where there is an insufficient number of hydrogen atoms to apply directly recommendations for the use of ‘ide’ given in P-72.2.2.1 is derived formally from a dihydro derivative of the cyclic parent hydride. Such an anion can also be described by applying the principle of ‘added indicated hydrogen’ (see P-14.7). In this method the ‘hydro’ derivative is described by specifying the hydrogen atom of a dihydro pair that remains after the anionic center is created by citing in italic capital H and the locant of the skeletal atom at which the hydrogen atom resides, both enclosed in a set of parentheses and inserted into the name of the corresponding parent hydride immediately after the locant for the anionic center. Names formed by the ‘added indicated hydrogen’ method are preferred IUPAC names (see P-58.2).
Examples:
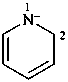
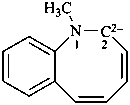
1-methyl-1-benzazocine-2,2(1H)-diide (PIN)
1-methyl-1,2-dihydro-1-benzazocine-2,2-diide
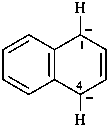
1,4-dihydronaphthalene-1,4-diide (PIN)
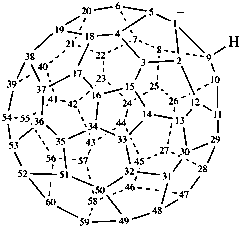
(C60-Ih)[5,6]fulleren-1(9H)-ide (PIN)
1,9-dihydro(C60-Ih)[5,6]fulleren-1-ide
(1) for acids, alcohols and amines by modifying the normally used in substitutive nomenclature:
(a) the endings ‘ate’ or ‘ite’ to name anions derived from acids;(2) by the appropriate preselected anionic parent names in the case of other characteristic groups, such as ‘azanide’ for NH2–, ‘azanediide’ for NH2–, ‘oxidanide’ for HO–.(b) the ending ‘ate’ to name anions derived from alcohols,
(c) the suffix ‘aminide’ (formed by adding ‘ide’ to the suffix of the corresponding amine with elision of the final ‘e’ of ‘amine’, i.e., ‘amin(e) + ide’) to name anions derived from amines where the negative charge is on the nitrogen atom;
(3) amides, hydrazides and imides are not named directly by method (1), as are amines and imines; the reason being that there could be real ambiguity to have the suffix ‘ide’ used at the end of names such as amide, hydrazides, etc.
Method (2) generates preferred names. Also, the name ‘amide’, which may be used in general nomenclature to designate the parent anion NH2–, would result in a certain degree of ambiguity. However, the use of parents ‘azanide’ and ‘azanediide’ eliminates all possible ambiguity.
P-72.2.2.2.1 Anions derived from acidsP-72.2.2.2.1 Anions derived from acids
P-72.2.2.2.2 Anions derived from hydroxy compounds
P-72.2.2.2.3 Anions derived from amines and imines
P-72.2.2.2.4 Anions derived from other characteristic groups
P-72.2.2.2.1.1 The preferred IUPAC name of anions formed by the removal of a hydron from the chalcogen atom (O, S, Se, and Te) of an acid or peroxyacid characteristic group or functional parent compound is formed by replacing the ‘ic acid’ or ‘ous acid’ ending of the acid name by ‘ate’ or ‘ite’, respectively. Names of acids are described in Sections P-65 and P-67.
This is a change from recommendation RC-83.1.6 (ref. 3) in which peroxyacids and their chalcogen analogues modified by functional replacement were named on the basis of an anionic parent hydride.
Examples:
CH3-CH2-CO-O-O–
propaneperoxoate (PIN)
CH3-CS-O-O–
ethaneperoxothioate (PIN)
(ethanethioyl)dioxidanide
(thioacetyl)dioxidanide
CH3-CO-O-S–
ethane(OS-thioperoxoate) (PIN)
(acetyloxy)sulfanide
(not acetoxysulfanide)
CH3-CH2-CO-S– ↔ CH3-CH2-CS-O–
propanethioate (PIN)
CH3-CO-S– ↔ CH3-CS-O–
ethanethioate (PIN)
C6H5-SO2-O–
benzenesulfonate (PIN)
(C6H5-CH2)2P-O–
dibenzylphosphinite (PIN)
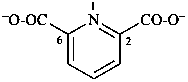
pyridine-2,6-dicarboxylate (PIN)
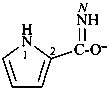
1H-pyrrole-2-carboximidate (PIN)
Preferred IUPAC names of acid esters of ‘organic acids’ as discussed in P-65 are formed substitutively (see P-65.6.3.3.5) rather than by the method of ‘hydrogen salts’. Preferred IUPAC names of acid esters of inorganic acids as discussed in P-67.1.3.2 are formed by the method of ‘hydrogen salts’; see P-65.6.2.3 and P-65.6.3.3.5.
Examples:
C6H5-P(O)(OH)-O–
hydrogen phenylphosphonate (PIN)
[not hydroxy(phenyl)phosphinate;
phosphonic acid is senior to phosphinic acid]
CH3-CH2-O-CO-CH2-CH2-CO-O–
4-ethoxy-4-oxobutanoate (PIN)
ethyl butanedioate
ethyl succinate
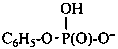
phenyl hydrogen phosphate (PIN)
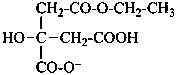
2-(carboxymethyl)-4-ethoxy-2-hydroxy-4-oxobutanoate (PIN)
4-ethyl 2-(carboxymethyl)-2-hydroxybutanedioate
3-ethyl 1-hydrogen citrate
4-hydrogen 2-(2-ethoxy-2-oxoethyl)-2-hydroxybutanedioate
An anion formed by subtracting a hydron from the chalcogen atom of a hydroxy characteristic group, or a chalcogen analogue, that can be expressed by a suffix such as ‘ol’, ‘thiol’, ‘peroxol’, etc., is preferably named by using suffixes ‘olate’, ‘thiolate’, ‘peroxolate’, etc., formed by addition of the ending ‘ate’ to the suffixes ‘ol’, ‘thiol’, ‘peroxol’, etc. The multiplying prefixes ‘bis’, ‘tris’, etc. are used before these suffixes, to avoid any ambiguity.
The retained names hydroxide, for HO–, and hydroperoxide, for HOO–, are preseleted names but cannot be substituted; thus, for CH3-O– and CH3-OO– the names are methoxide or methanolate or methyloxidanide, and methaneperoxolate or methyldioxidanide, respectively.
The traditional names methoxide, ethoxide, propoxide, butoxide, tert-butoxide, phenoxide (but not isopropoxide), and aminoxide, for CH3-O–, C2H5-O–, C3H7-O–, C4H9-O–, (CH3)3C-O–, C6H5-O–, and H2N-O–, are retained as preferred IUPAC names or preselected name. tert-Butoxide cannot be substituted. Isopropoxide, (CH3)2CH-O–, is retained for general nomenclature but cannot be substituted.
Examples:
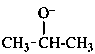
propan-2-olate (PIN)
isopropoxide
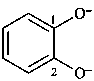
benzene-1,2-bis(olate) (PIN)
(not pyrocatecholate)
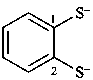
benzene-1,2-bis(thiolate) (PIN)
(CH3)2N-O–
dimethylaminoxide (PIN)
(dimethylamino)oxidanide
CH3-O-O–
methaneperoxolate (PIN)
methyldioxidanide
CH3-CH2-S-O–
ethane(SO-thioperoxolate) (PIN)
(ethylsulfanyl)oxidanide
(not ethanesulfenate;
sulfenic acids are no longer recommended; see P-56.2)
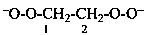
ethane-1,2-bis(peroxolate) (PIN)
(ethane-1,2-diyl)bis(dioxidanide)
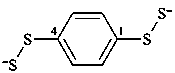
benzene-1,4-bis(dithioperoxolate) (PIN)
(1,4-phenylene)bis(disulfanide)
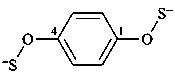
benzene-1,4-bis(OS-thioperoxolate) (PIN)
(1,4-phenylene)bis(oxy)bis(sulfanide)
Amines and imines having one negative charge on each nitrogen atom are named by using the suffixes ‘aminide’ and ‘iminide’, formed by the addition of the suffix ‘ide’ to the suffix ‘amine’ or ‘imine’, respectively; the prefix ‘bis-’ is used to indicate two aminide suffixes. The resulting names are preferred IUPAC names. When two negative charges are present on the nitrogen atom of an amine, the suffix ‘aminediide’ is used to generate preferred IUPAC names. The retained names ‘amide’ and ‘imide’ for the anions H2N– and HN2–, respectively, may be used as parent anions in general nomenclature.
The use of compound suffixes ‘aminide’, ‘iminide’ and ‘aminediide’ is a change from previous practice where parent anions H2N– and HN2– were used to express amines and imines having negative charge(s).
Examples:
C6H5-NH–
benzenaminide (PIN)
phenylamide
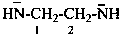
ethane-1,2-bis(aminide) (PIN)
(ethane-1,2-diyl)bis(amide)
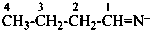
butaniminide (PIN)
(CH3)3P=N–
trimethyl-λ5-phosphaniminide (PIN)
CH3-CH2-N2–
ethanaminediide (PIN)
ethylazanediide
ethylimide
C6H5-N2–
benzenaminediide (PIN)
phenylazanediide
phenylimide
Anionic centers generated formally by the removal of hydrons from atoms of characteristic groups other than those considered in P-72.2.2.2.1, P-72.2.2.2.2, and P-72.2.2.2.3 are named on the basis of the corresponding anionic parent hydrides. Suffixes such as ‘amidide’ and ‘carboxamidide’ are not recommended.
Examples:
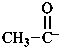
1-oxoethan-1-ide (PIN)
CH3-CO-NH–
acetylazanide (PIN)
acetylamide
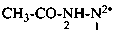
acetylhydrazine-1,1-diide (PIN)
acetyldiazane-1,1-diide
HO-NH–
hydroxyazanide (preselected name)
hydroxyamide
HO-N2–
hydroxyazanediide (preselected name)
hydroxyimide
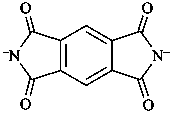
1,3,5,7-tetraoxo-5,7-dihydrobenzo[1,2-c:4,5-c′]dipyrrole-2,6(1H,3H)-diide (PIN)
(not 1,3,5,7-tetraoxo-5,7-dihydropyrrolo[3,4-f]isoindole-2,6(1H,3H)-diide;
a multiparent system is preferred to a two-component fused system, see P-25.3.5.3)
Two methods are used to name anions formally derived by the addition of a hydride ion, H–:
(1) the suffix ‘uide’ describes an anion formally derived by adding a hydride ion, H–, to a parent hydride name; the multiplying prefixes ‘di’, ‘tri’, etc. indicating multiplicity;(2) by adding the suffix ‘ide’ to a parent hydride in which the bonding number at the anionic site is higher than the standard bonding number and is expressed by the λ-convention; the net effect of which is the addition of a hydride ion, H–, to the parent hydride with its standard bonding number (see P-72.2.2.1).
In previous recommendations, the skeletal replacement prefix ‘borata’ was used to describe the addition of a hydride ion.
Method (1) leads to preferred IUPAC names.
Examples:
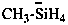
(CH3)4B–
tetramethylboranuide (PIN)
(CH3)4P–
tetramethylphosphanuide (PIN)
tetramethyl-λ5-phosphanide
tetramethylphosphoranide
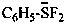
difluoro(phenyl)sulfanuide (PIN)
difluoro(phenyl)-λ4-sulfanide
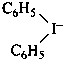
diphenyliodanuide (PIN)
diphenyl-λ3-iodanide
F6I–
hexafluoro-λ5-iodanuide (preselected name)
hexafluoro-λ7-iodanide
F8Te2–
octafluoro-λ6-tellanediuide (preselected name)
octafluoro-λ10-tellanediide
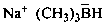
sodium trimethylboranuide (PIN)
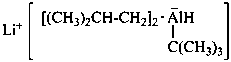
lithium tert-butylbis(2-methylpropyl)alumanuide
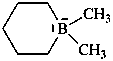
1,1-dimethylborinan-1-uide (PIN)
(not 1,1-dimethyl-1-boratacyclohexane)
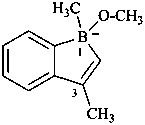
1-methoxy-1,3-dimethyl-1H-1-benzoborol-1-uide (PIN)
(not 1-methoxy-1,3-dimethyl-1-borataindene)
Anionic centers in parent hydrides are named by two methods using the principles of skeletal replacement (‘a’) nomenclature described in Section P-15.4:
(1) by forming the name of the neutral compound according to skeletal replacement (‘a’) nomenclature and using the suffixes ‘ide’ and ‘uide’ to describe the anionic centers;Method (1) results in preferred IUPAC names. In other words, names that do not require designation of skeletal heteroatoms in nonstandard valence states using the λ-convention are preferred (see P-72.3).(2) by adding anionic skeletal replacement (‘a’) prefixes formed by adding the suffixes ‘ida’ and ‘uida’ to the name of the corresponding mononuclear parent hydride, with elision of the final letter ‘e’; these replacement prefixes indicate an anionic center having a bonding number one lower or one higher, respectively, than the bonding number of the corresponding neutral mononuclear parent hydride.
Skeletal replacement (‘a’) prefixes ending in ‘ata’, for example ‘borata’, are no longer recognized.
Examples:
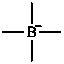
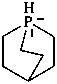
boranuida (preselected name)
(not borata)
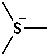
sulfanuida (preselected name)
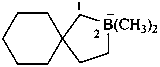
6λ5-phosphaspiro[5.5]undecan-6-uide (PIN)
6λ5-phosphanuidaspiro[5.5]undecane
(not 6-phosphataspiro[5.5]undecane)
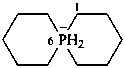
1-phosphabicyclo[2.2.2]octan-1-uide (PIN)
1-phosphanuidabicyclo[2.2.2]octane
1λ5-phosphabicyclo[2.2.2]octan-1-ide
1λ5-phosphanidabicyclo[2.2.2]octane
Multiple anionic centers are named by several methods in accordance with the previous rules.
P-72.5.1 Assemblies of parent anionsP-72.5.1 Assemblies of parent anions
P-72.5.2 ‘Ide’ and ‘uide’ centers in the same parent hydride
P-72.5.3 Anionic characteristic groups on anionic parent hydrides
P-72.5.1.1 Assemblies derived from parent anions
Anionic compounds with anionic centers derived from the same parent hydride, but located in different parts of a structure, are named, if possible, according to the principles of multiplicative nomenclature (see P-15.3), using the multiplying prefixes ‘bis’, ‘tris’, etc., where necessary.
Examples:
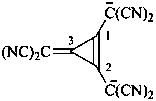
[3-(dicyanomethylidene)cycloprop-1-ene-1,2-diyl]bis(dicyanomethanide) (PIN)
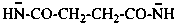
butanedioylbis(azanide) (PIN)
butanedioylbis(amide)
Anionic compounds with two or more anionic centers in the same parent hydride structure, at least one of which is derived formally by removal of a hydron from a skeletal position and one by adding a hydride ion at another position, are named by adding the suffix ‘-ide’, then the suffix ‘-uide’ to the name of the parent hydride, with elision of the final letter ‘e’ of the parent hydride and of the suffix ‘-ide’. Each suffix is preceded, where necessary, by the appropriate multiplying prefix. Where there is a choice, low locants of the parent hydride are assigned first to the anionic centers regardless of the kind and then to ‘-uide’ anionic centers.
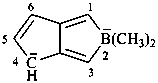
Example:
Polyanions with anionic centers both in the parent hydride part of the structure and on a characteristic group that may be expressed as an anionic suffix are named by adding the anionic suffix to the name of a parent anion formed according to Rules P-72.2.2.1 and P-72.2.2.2. Where there is a choice, low locants are assigned to the anionic skeletal atoms.
Examples:
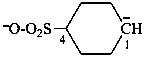
–O-CO-CH2-CH2-C≡C–
pent-1-yn-1-id-5-oate (PIN)
When anionic centers are not in the same parent structure, one anion must be chosen as the parent anion and the other expressed as anionic substituent group(s).
P-72.6.1 Prefixes for anionic centers derived from acid characteristic groupsP-72.6.1 Prefixes for anionic centers derived from acid characteristic groups
P-72.6.2 Prefixes for anionic chalcogens
P-72.6.3 Systematically formed prefixes that include anionic center(s)
Substituent anions derived from acid characteristic groups by removal of a hydron from all hydroxy, thiol, etc. groups or a chalcogen analogue, and that are attached to the parent structure by a single bond are named by prefixes formed by changing the ending ‘ate’ in the name of the anionic suffix to ‘ato’.
Examples:
–SO2-O–
sulfonato (preselected prefix)
–P(O)(O–)2
phosphonato (preselected prefix)
–As(O)(O–)2
arsonato (preselected prefix)
These prefixes are derived from the names oxide, sulfide, selenide, and telluride by changing the final letter ‘e’, to ‘o’.
Examples:
–S–
sulfido (preselected prefix)
These prefixes are formed by adding the cumulative suffixes ‘yl’ or ‘ylidene’ to the name of the parent anion, with elision of the final letter ‘e’ in the name of the parent anion. Multiplying prefixes ‘di’, ‘tri’, etc, are used to denote multiplicity of free valences. Where there is a choice, low locants are assigned to the free valences.
Examples:
–NH–
azanidyl (preselected prefix)
amidyl
–N2–
azanediidyl (preselected prefix)
–BH3–
boranuidyl (preselected prefix)
=N–
azanidylidene (preselected prefix)
amidylidene
–S-S–
disulfanidyl (preselected prefix)
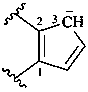
cyclopenta-1,4-dien-3-ide-1,2-diyl (preferred prefix)
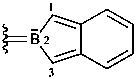
2H-2-benzoborol-2-uid-2-ylidene (preferred prefix)
When necessary, a parent anionic structure must be chosen by applying the following criteria in order until a definitive choice is achieved:
(a) parent with the maximum number of anionic centers, including anionic suffixes;
Example:
Example:
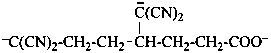
Example:
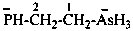
The seniority order for anions is now the order of seniority of classes rather than the order of skeletal replacement (‘a’) prefixes as used in RC-83.4.7.4, ref. 3.
Example:
Examples:
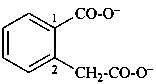
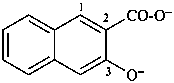
3-oxidonaphthalene-2-carboxylate (PIN)
(carboxylate senior to olate)
P-72.8.1 The suffix ‘uide’ is preferred to the suffix ‘ide’ with a parent hydride named by the λ-convention.
Examples:
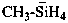
F6I–
hexafluoro-λ5-iodanuide (preselected name)
hexafluoro-λ7-iodanide
Examples:
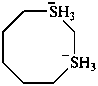
1λ6,3λ6-dithiocane-1,3-diide (PIN)
1λ4,3λ4-dithiocane-1,3-diuide
(not 1λ4,3λ6-dithiocan-3-id-1-uide;
identical groups, if possible, must not be named differently within a name)
P-73.0 INTRODUCTION
For the purpose of organic nomenclature a cation is a molecular entity carrying at least one unit of positive charge formally derived from a parent hydride or parent compound by adding one or more hydrons, by the removal of one or more hydride ions, or a combination of these operations. An atom where a positive charge is considered to reside is called a cationic center. Cations with two or more cationic centers in the same structure are called dications, trications, etc.
P-73.1 Cationic compounds with cationic centers derived formally by the addition of hydronsP-73.1 CATIONIC COMPOUNDS WITH CATIONIC CENTERS DERIVED FORMALLY BY THE ADDITION OF HYDRONS
P-73.2 Cationic compounds with cationic centers derived formally by the removal of hydride ions
P-73.3 The λ-convention with the suffix ‘ylium’
P-73.4 Skeletal replacement (‘a’) nomenclature for cations
P-73.5 Cationic compounds with multiple cationic centers
P-73.6 Cationic prefix names
P-73.7 Choice of a parent structure
P-73.8 The suffixes ‘ium’ and ‘ylium’ and the λ-convention
P-73.1.1 Cationic centers in parent hydridesP-73.1.1 Cations centers in parent hydrides
P-73.1.2 Cationic centers on characteristic groups
P-73.1.1.1 Retained names for monocationic mononuclear parent cations of the Group 15, 16, and 17 elements used only for general nomenclature.
A parent ion derived formally by adding one hydron to a mononuclear parent hydride of the nitrogen, chalcogen, and halogen families in its standard bonding state is named by adding the term ‘onium’ to a root for the element as indicated in Table 7.3. These cations are parent compounds; they can be substituted, but are used only in general nomenclature. For preferred IUPAC names see P-73.1.1.2.
| H4N+ | ammonium | H3O+ | oxonium | H2F+ | fluoronium |
| H4P+ | phosphonium | H3S+ | sulfonium | H2Cl+ | chloronium |
| H4As+ | arsonium | H3Se+ | selenonium | H2Br+ | bromonium |
| H4Sb+ | stibonium | H3Te+ | telluronium | H2I+ | iodonium |
| H4Bi+ | bismuthonium |
Examples:
Cl(CH3)3P+
chlorotri(methyl)phosphonium
chlorotri(methyl)phosphanium (PIN)
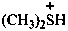
dimethylsulfonium
dimethylsulfanium (PIN)
CH3-C≡O+
ethylidyneoxonium
ethylidyneoxidanium(PIN)
(C6H5)2I+
diphenyliodonium
diphenyliodanium (PIN)
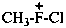
chloro(methyl)fluoronium
chloro(methyl)fluoranium (PIN)
A cation derived formally by adding one or more hydrons to any position of a neutral parent hydride (listed in Chapter P-2), or whose degree of hydrogenation has been modified (see P-31) is named by replacing the final letter ‘e’ of the parent hydride name, if any, by the suffix ‘ium’, preceded by multiplying prefixes ‘di’, ‘tri’, etc. to denote the multiplicity of identical cationic centers. These names for mononuclear cations derived from the mononuclear parent hydrides of the Group 15, 16, and 17 elements are the preferred IUPAC names and not those given in Table 7.3. When the hydron is not specifically localized the structure is enclosed in square brackets.
Examples:
[C6H7]+
benzenium (PIN)
H4P+
phosphanium (preselected name)
phosphonium
H3S+
sulfanium (preselected name)
sulfonium
H2Cl+
chloranium (preselected name)
chloronium
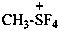
tetrafluoro(methyl)-λ4-sulfanium (PIN)
tetrafluoro(methyl)-λ4-sulfonium
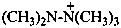
pentamethylhydrazinium (PIN)
pentamethyldiazanium
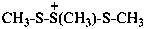
1,2,3-trimethyltrisulfan-2-ium (PIN)
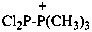
2,2-dichloro-1,1,1-trimethyldiphosphan-1-ium (PIN)
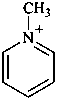
1-methylpyridin-1-ium (PIN)
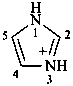
1H-imidazol-3-ium (PIN)
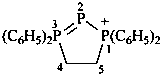
1,1,3,3-tetraphenyl-4,5-dihydro-1H-1,2,3λ5-triphosphol-1-ium (PIN)
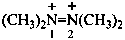
tetramethyldiazene-1,2-diium (PIN)
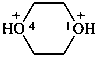
1,4-dioxane-1,4-diium (PIN)
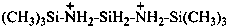
N,N′-bis(trimethylsilyl)silanebis(aminium) (PIN)
The principle applied in the naming of cationic centers on characteristic groups is that the largest neutral parent possible is used. It is applied particularly in the case of neutral compounds expressed by suffixes containing nitrogen (see Table 7.4, below). Other classes are named on the basis of the largest cationic parent hydride.
| Neutral characteristic group suffix | Cationic characteristic group suffix |
| amide, carboxamide | amidium, carboxamidium |
| imide, carboximide | imidium, carboximidium |
| nitrile, carbonitrile | nitrilium, carbonitrilium |
| amine | aminium |
| imine | iminium |
| 1 When retained names of amides and nitriles used in general nomenclature imply the presence of two characteristic groups, for example succinonitrile, the corresponding cationic suffix denotes the addition of one hydron to each of the characteristic groups. | |
P-73.1.2.1 Cationic compounds derived from neutral compounds expressed by suffixes are named in two ways.
(1) Cationic suffixes derived from names of acids named by a suffix, amides, imides, nitriles, amines, and imines are formed by adding the suffix ‘ium’ to the basic suffix, as indicated in Table 7.4. These cationic suffixes are used with the multiplying prefixes ‘bis’, ‘tris’, etc. to denote multiplicity. Retained names, whether used as preferred IUPAC names or in general nomenclature (with the exception of ‘urea’, see P-73.1.2.2), are modified by adding the suffix ‘ium’ to the name of the neutral entity.Methods (1) and (2) are used to generate preferred IUPAC names as illustrated by the examples below.(2) by substituting cationic parent hydrides described in P-73.1.2 when no nitrogen atom is present or when they are nitrogen containing compounds other than those described in (1).
Examples:
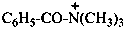
N,N,N-trimethylbenzamidium (PIN)
benzoyltri(methyl)ammonium
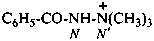
N′,N′,N′-trimethylbenzohydrazid-N′-ium (PIN)
2-benzoyl-1,1,1-trimethylhydrazinium
2-benzoyl-1,1,1-trimethyldiazan-1-ium
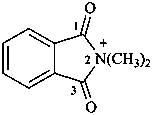
2,2-dimethyl-1,3-dioxo-2,3-dihydro-1H-isoindol-2-ium (PIN)
N,N-dimethylphthalimidium
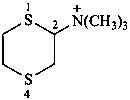
N,N,N-trimethyl-1,4-dithian-2-aminium (PIN)
(1,4-dithian-2-yl)tri(methyl)ammonium
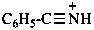
benzonitrilium (PIN)
benzylidyneammonium
benzylidyneazanium
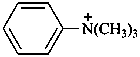
N,N,N-trimethylanilinium (PIN)
N,N,N-trimethylbenzenaminium
trimethyl(phenyl)ammonium
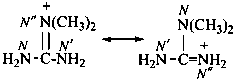
N,N-dimethylguanidinium (PIN)
(locants ‘N,N’ are lower than ‘N′,N′’ in designating these resonance forms)
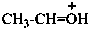
ethylideneoxidanium (PIN)
ethylideneoxonium
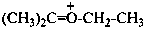
ethyl(propan-2-ylidene)oxidanium (PIN)
ethyl(propan-2-ylidene)oxonium
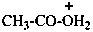 |  | 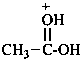 |
| acetic acidium | ||
| acetyloxidanium (PIN) | (1-hydroxyethylidene)oxidanium (PIN) | |
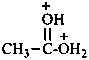
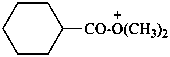
(cyclohexanecarbonyl)di(methyl)oxidanium (PIN)
(cyclohexanecarbonyl)di(methyl)oxonium
(not O,O-dimethylcyclohexanecarboxylic acidium
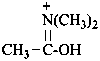
(1-hydroxyethylidene)di(methyl)azanium (PIN)
(1-hydroxyethylidene)di(methyl)ammonium
(not N,N-dimethylethanimidic acidium)
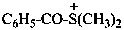
benzoyldi(methyl)sulfanium (PIN)
benzoyldi(methyl)sulfonium
(not S,S-dimethylbenzenecarbothioic acidium)
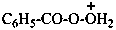
2-benzoyldioxidan-1-ium (PIN)
(not peroxybenzoic OO-acidium)
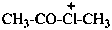
acetyl(methyl)chloranium (PIN)
Cations derived formally by adding a hydron to urea (or isourea) are named on the basis of the parent cation ‘uronium’, representing the following tautomeric structures:

Numerical locants for the parent cation uronium are no longer used in preferred IUPAC names.
Locants follow those used for urea and isourea. Chalcogen analogues are named on the basis of parent cations, such as ‘thiouronium’, etc. This methodology leads to preferred IUPAC names.
Examples:
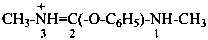
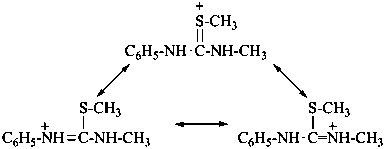
N,S-dimethyl-N′-phenylthiouronium (PIN)
P-73.2.1 Functional class namesP-73.2.1 Functional class names
P-73.2.2 Cationic centers in parent hydrides
P-73.2.3 Cationic centers on characteristic groups
Cationic compounds that can be considered as being derived formally by removal of electrons from the corresponding radical may be named by adding the class name ‘cation’ as a separate word after the name of the radical. Polycations are indicated by adding the multiplying prefixes ‘di’, ‘tri’, etc., as appropriate, to the class name. Systematic names formed by using the suffix ‘ylium’ are preferred IUPAC names (see P-73.2.2). When the charge is not localized, the structure is enclosed in square brackets.
Examples:
[C6H5]+
phenyl cation
phenylium
benzenylium (PIN)
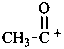
acetyl cation
acetylium (PIN)
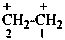
ethane-1,2-diyl dication
ethane-1,2-bis(ylium) (PIN)
The following recommendations follow closely those for naming radicals, for which see P-71.
P-73.2.2.1 Cationic centers in parent hydridesP-73.2.2.1 Cationic centers in parent hydrides
P-73.2.2.2 ‘Added indicated hydrogen’ for cations of mancude ring systems
P-73.2.2.3 Diazonium ions
P-73.2.2.1.1 Specific method
Cations formed formally by the removal of a hydride ion, H–, from a terminal atom of a saturated unbranched acyclic hydrocarbon, a saturated monocyclic hydrocarbon, or a mononuclear parent hydride belonging to Group 14, i.e., methane, CH4, silane, SiH4, germane, GeH4, stannane, SnH4, and plumbane, PbH4, are named by replacing the ‘ane’ ending in the name of the parent hydride by the suffix ‘ylium’.
Examples:
(C6H5)3Si+
triphenylsilylium (PIN)
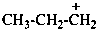
propylium (PIN)
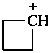
cyclobutylium (PIN)
According to the general method, cations formally derived by the removal of one hydride ion, H–, from any position of a parent hydride are named by adding the suffix ‘-ylium’ to the name of the parent hydride, with elision of the final ‘e’ in the name of the parent hydride, if present. Di- and polycations formally derived by the removal of two or more hydride ions from the parent hydride are named by using the suffix ‘ylium’ and the multiplying prefixes ‘bis’, ‘tris’, etc. Preferred IUPAC names for the parent hydrides are used, as indicated in Chapters P-2 and P-5. In the examples that follow, preferred IUPAC names are indicated when traditional names are used in general nomenclature.
Examples:
C6H5-S+
phenylsulfanylium (PIN)
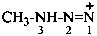
3-methyltriaz-1-en-1-ylium (PIN)
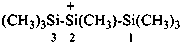
heptamethyltrisilan-2-ylium (PIN)
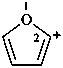
furan-2-ylium (PIN)
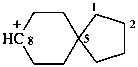
spiro[4.5]decan-8-ylium (PIN)
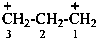
propane-1,3-bis(ylium) (PIN)
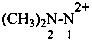
2,2-dimethylhydrazine-1,1-bis(ylium) (PIN)
2,2-dimethyldiazane-1,1-bis(ylium)
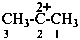
propane-2,2-bis(ylium) (PIN)
1-methylethane-1,1-bis(ylium)
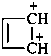
cyclobut-3-ene-1,2-bis(ylium) (PIN)
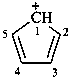
cyclopenta-2,4-dien-1-ylium (PIN)
cyclopentadienylium (see P-76)
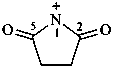
2,5-dioxopyrrolidin-1-ylium (PIN)
succinimidylium
A cationic center at a position in a mancude parent hydride where there is an insufficient number of hydrogen atoms to apply directly recommendations for the use of ‘ylium’ as given in P-73.2.2.1.2 is derived formally from a dihydro derivative of the cyclic parent hydride. Such a cation can also be described by applying the principle of ‘added indicated hydrogen’ (see P-14.7). In this method the ‘hydro’ derivative is described by specifying the hydrogen atom of a dihydro pair that remains after the cationic center is created by citing an italic capital H and the locant of the skeletal atom at which the hydrogen atom resides, both enclosed in a set of parentheses and inserted into the name of the corresponding parent hydride immediately after the locant for the cationic center. Names formed by the ‘added indicated hydrogen’ method are preferred IUPAC names (see P-58.2).
Examples:
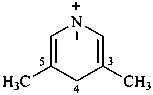
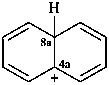
naphthalen-4a(8aH)-ylium (PIN)
4a,8a-dihydronaphthalen-4a-ylium
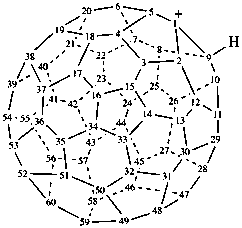
(C60-Ih)[5,6]fulleren-1(9H)-ylium (PIN)
1,9-dihydro(C60-Ih)[5,6]fulleren-1-ylium
Cations containing an –N2+ group attached to a parent hydride are traditionally named according to the principles of substitutive nomenclature by using the suffix ‘diazonium’ and the multiplying prefixes ‘bis’, ‘tris’, etc. to denote multiplicity. Diazonium ions may also be named on the basis of the parent cation ‘diazenylium’, HN=N+. The use of the suffix ‘diazonium’ results in preferred IUPAC names.
Examples:
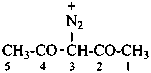
2,4-dioxopentane-3-diazonium (PIN)
(2,4-dioxopentan-3-yl)diazenylium
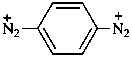
benzene-1,4-bis(diazonium) (PIN)
(1,4-phenylene)bis(diazenylium)
P-73.2.3.1 Acylium cations
Cations formally derived by the removal of all the hydroxy groups as hydroxide ions from acids having systematic or retained names are named by replacing the ‘oic acid’ or ‘ic acid’ ending by the suffix ‘oylium’ or ‘ylium’, or the ‘carboxylic acid’ ending by ‘carbonylium’, in accordance with the rules for naming neutral acyl groups (see P-65.1.7). These names are preferred IUPAC names.
Examples:
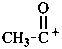
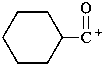
cyclohexanecarbonylium (PIN)
cyclohexanecarbonyl cation
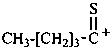
pentanethioylium (PIN)
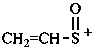
ethenesulfinylium (PIN)
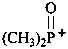
dimethylphosphinoylium (PIN)
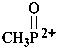
methylphosphonobis(ylium) (PIN)
Examples:
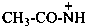
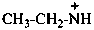
ethanaminylium (PIN)
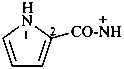
1H-pyrrole-2-carboxamidylium (PIN)
(1) additively, using the term ‘oxylium’ or ‘peroxylium’;The names methoxylium, ethoxylium, propoxylium, butoxylium and phenoxylium are retained as preferred IUPAC names, and aminoxylium as a preselected name.(2) by substituting the parent cations ‘oxidanylium’ or ‘dioxidanylium’ (preselected names) by the appropriate substituent groups.
Examples:
(CH3)3C-O-O+
tert-butylperoxylium (PIN)
tert-butyldioxidanylium
Cl-CH2-CO-O+
(chloroacetyl)oxylium (PIN)
chloroacetoxylium
(chloroacetyl)oxidanylium
CH3-CS-O+
(ethanethioyl)oxylium (PIN)
(ethanethioyl)oxidanylium
(CH3)2N-O+
N-methylmethanaminoxylium (PIN)
(dimethylamino)oxidanylium
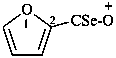
(furan-2-carboselenoyl)oxylium (PIN)
(furan-2-carboselenoyl)oxidanylium
All other cationic centers are named by substituting the appropriate parent cation. In the case of sulfur cationic centers, the terms ‘thioxylium’ and ‘dithioperoxylium’, but not the term ‘thioperoxylium’ (ambiguity), may be used in general nomenclature. Use of the terms ‘thiylium’ or ‘perthiylium’ is not recommended.
Examples:
CH3-CO-S+
acetylsulfanylium (PIN)
acetylthioxylium
(not acetylthiylium)
C6H5-S-S+
phenyldisulfanylium (PIN)
phenyldithioperoxylium
(not phenylperthiylium)
CH3-CH2-S-O+
(ethylsulfanyl)oxylium (PIN)
(ethylsulfanyl)oxidanylium
[not (ethylsulfanyl)thioperoxylium]
CH3-CH2-N2+
ethylazanebis(ylium) (PIN)
[(not ethanaminebis(ylium)]
C6H5-CO-N2+
benzoylazanebis(ylium) (PIN)
[not benzamidebis(ylium)]
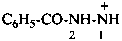
2-benzoylhydrazin-1-ylium (PIN)
2-benzoyldiazan-1-ylium
(not benzohydrazid-N′-ium)
CH3-CH2-O-Te+
ethoxytellanylium (PIN)
(not ethyltelluroperoxylium)
P-73.3.1 Application of the λ-convention with the suffix ‘ylium’
Cationic heterocycle having a cationic center on a heteroatom that has one more skeletal bonds than it has in the corresponding neutral heterocycle is named by adding the suffix ‘ylium’ to the name of the neutral parent hydride for which the λ-convention has been used to describe a nonstandard bonding state of the heteroatom, and that heteroatom has at least one hydrogen atom in the neutral heterocycle on which the ‘ylium’ suffix can operate. Indicated hydrogen (see P-14.7 and P-58.2.1) is used as needed.
Examples:
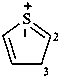
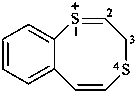
3H-1λ4,4-benzodithiocin-1-ylium (PIN)
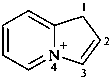
1H-4λ5-indolizin-4-ylium (PIN)
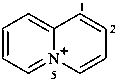
5λ5-quinolizin-5-ylium (PIN)
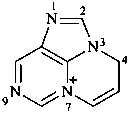
4H-7λ5-pyrimido[1,2,3-cd]purin-7-ylium (PIN)
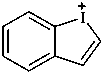
1λ3-benziodol-1-ylium (PIN)
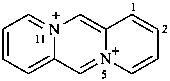
5λ5,11λ5-dipyrido[1,2-a:1′,2′-d]pyrazine-5,11-bis(ylium) (PIN)
Example:
Example:
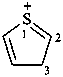
The contracted and traditional names listed in Table 7.5 are retained as preferred IUPAC names and for use in general nomenclature.
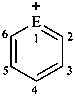 |  | |||
| E = O | pyrylium (PIN) | E = O | 1λ4-benzopyran-1-ylium (PIN) chromenylium | |
| E = S | thiopyrylium (PIN) | E = S | 1λ4-benzothiopyran-1-ylium (PIN) thiochromenylium | |
| E = Se | selenopyrylium (PIN) | E = Se | 1λ4-benzoselenopyran-1-ylium (PIN) selenochromenylium | |
| E = Te | telluropyrylium (PIN) | E = Te | 1λ4-benzotelluropyran-1-ylium (PIN) tellurochromenylium | |
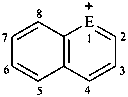
 | E = O | 2λ4-benzopyran-2-ylium (PIN) isochromenylium |
| E = S | 2λ4-benzothiopyran-2-ylium (PIN) isothiochromenylium | |
| E = Se | 2λ4-benzoeselenopyran-2-ylium (PIN) isoselenochromenylium | |
| E = Te | 2λ4-benzotelluropyran-2-ylium (PIN) isotellurochromenylium | |
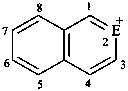
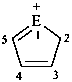 | E = O | 2H-1λ4-furan-1-ylium (PIN) |
| E = S | 2H-1λ4-thiophen-1-ylium (PIN) | |
| E = Se | 2H-1λ4-selenophen-1-ylium (PIN) | |
| E = Te | 2H-1λ4-tellurophen-1-ylium (PIN) | |
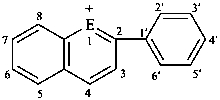 | 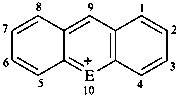 | |||
| E = O | flavylium | E = O | xanthylium (PIN) | |
| E = S | thioflavylium | E = S | thioxanthylium (PIN) | |
| E = Se | selenoflavylium | E = Se | selenoxanthylium (PIN) | |
| E = Te | telluroflavylium | E = Te | telluroxanthylium (PIN) | |
P-73.4 SKELETAL REPLACEMENT (‘a’) NOMENCLATURE FOR CATIONS
Two methods are used to name cationic centers by skeletal replacement (‘a’) nomenclature:
(1) name the compound using neutral skeletal replacement (‘a’) prefixes and then describe the cationic centers by the appropriate suffix ‘ium’ and ‘ylium’;Cationic skeletal replacement (‘a’) prefixes to indicate a cationic center having a bonding number one higher than the bonding number of the corresponding neutral mononuclear hydride, except for bismuth, are formed by replacing the ‘a’ ending of the normal skeletal replacement (‘a’) prefixes by ’onia’; the cationic skeletal replacement (‘a’) prefix corresponding to ‘bismuthonium’ is ‘bismuthonia’.(2) by using cationic skeletal replacement (‘a’) prefixes.
Cationic skeletal replacement (‘a’) prefixes to indicate a cationic center having a bonding number one lower than the bonding number of the corresponding neutral mononuclear hydride, except for carbon, are formed by replacing the final ‘e’ of the name of the fundamental parent hydride by ‘ylia’.
Cationic skeletal replacement (‘a’) prefixes are used in the same way as neutral replacement prefixes.
Examples:
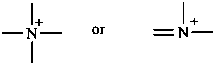
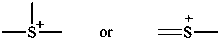
thionia (preselected name)
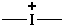
iodonia (preselected name)
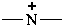
azanylia (preselected name)
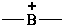
boranylia (preselected name)
Examples:
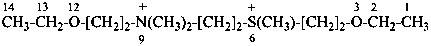
2-ethoxy-N-{2-[(2-ethoxyethyl)(methyl)sulfaniumyl]ethyl}-N,N-dimethylethanaminium (PIN)
{not 6,9,9-trimethyl-3,12-dioxa-6-thia-9-azatetradecane-6,9-diium;
nor 6,9,9-trimethyl-3,12-dioxa-6-thionia-9-azoniatetradecane;
the name must be based on a cation derived from the preferred amine name which is
2-ethoxy-N-{2-[(2-ethoxyethyl)(methyl)sulfanyl]ethyl}-N-methylethanamine and
not N-(2-ethoxyethyl)-2-[(2-ethoxyethyl)(methyl)sulfanyl]-N-methylethanamine
which is preferred alphanumerically, but does not qualify for a skeletal replacement (‘a’) name because it does not have four heterounits}
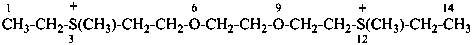
3,12-dimethyl-6,9-dioxa-3,12-dithiatetradecane-3,12-diium (PIN)
3,12-dimethyl-6,9-dioxa-3,12-dithioniatetradecane
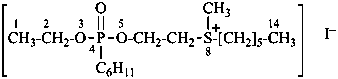
4-cyclohexyl-8-methyl-4-oxo-3,5-dioxa-8-thia-4λ5-phosphatetradecan-8-ium iodide (PIN)
4-cyclohexyl-8-methyl-4-oxo-3,5-dioxa-8-thionia-4λ5-phosphatetradecane iodide
(2-{[cyclohexyl(ethoxy)phosphinoyl]oxy}ethyl)hexyl(methyl)sulfonium iodide
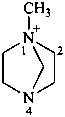
1-methyl-1,4-diazabicyclo[2.2.1]heptan-1-ium (PIN)
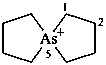
5λ5-arsaspiro[4.4]nonan-5-ylium (PIN)
5-arsoniaspiro[4.4]nonane
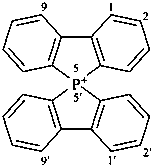
5H-5λ5,5′-spirobi[benzo[b]phosphindol]-5-ylium (PIN)
9-phosphonia-9,9′-spirobi[fluorene];
for construction of the name of the corresponding neutral, noncationic compound, see P-24.8.2)
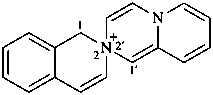
1H-2λ5-spiro[isoquinoline-2,2′-pyrido[1,2-a]pyrazin]-2-ylium (PIN)
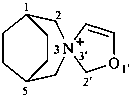
2′H-3λ5-spiro[3-azabicyclo[3.2.2]nonane-3,3′-[1,3]oxazol]-3-ylium (PIN)
Cationic compounds with multiple cationic centers are named by several methods in addition to previous rules.
P-73.5.1 Assemblies of parent cationsP-73.5.1 Assemblies of parent cations
P-73.5.2 ‘Ium’ and ‘ylium’ centers in the same parent hydride
P-73.5.3 Cationic characteristic groups on parent cations
P-73.5.1.1 Assemblies derived from parent cations
Cationic compounds with cationic centers derived from the same parent hydride, but located in different parts of a structure, are named, if possible, according to the principles of multiplicative nomenclature (see P-15.3), using the multiplying prefixes ‘bis-’, ‘tris-’, etc. where necessary.
Examples:
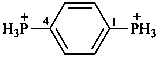
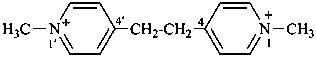
4,4′-(ethane-1,2-diyl)bis(1-methylpyridin-1-ium) (PIN)
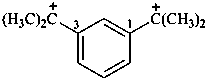
2,2′-(1,3-phenylene)di(propan-2-ylium) (PIN)
Polycations with cationic centers on characteristic groups are named by substitutive nomenclature or multiplicative nomenclature using the multiplying prefixes ‘bis-’, ‘tris-’, etc.
Examples:
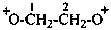
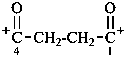
1,4-dioxobutane-1,4-bis(ylium) (PIN)
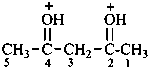
(pentane-2,4-diylidene)bis(oxidanium) (PIN)
(pentane-2,4-diylidene)bis(oxonium)
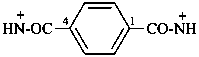
benzene-1,4-bis(carboxamidylium) (PIN)
(1,4-phenylenedicarbonyl)bis(azanylium)
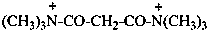
N1,N1,N1,N3,N3,N3-hexamethylpropanebis(amidium) (PIN)
N,N,N,N′,N′,N′-hexamethylmalonamidium
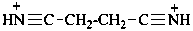
butanebis(nitrilium) (PIN)
butanediylidynebis(ammonium)
butanediylidynebis(azanium)
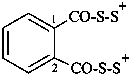
(benzene-1,2-dicarbonyl)bis(disulfanylium) (PIN)
(1,2-phenylenedicarbonyl)bis(disulfanylium)
(1,2-phenylenedicarbonyl)bis(dithioperoxylium)
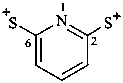
(pyridine-2,6-diyl)bis(sulfanylium) (PIN)
(pyridine-2,6-diyl)bis(thioxylium)
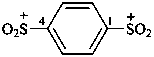
benzene-1,4-bis(sulfonylium)
(1,4-phenylene)bis(dioxo-λ6-sulfanylium) (PIN)
Examples:
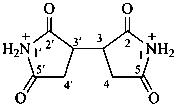
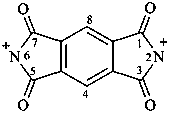
1,3,5,7-tetraoxo-5,7-dihydrobenzo[1,2-c:4,5-c′]dipyrrole-2,6(1H,3H)-bis(ylium) (PIN)
Cyclic compounds with two or more cationic centers in the same parent hydride structure at least one of which being denoted by ‘ium’ and another by ‘ylium’ suffixes, are named by placing the ‘ium’ and ‘ylium’ suffixes, in that order, after the name of the parent hydride, preceded by the appropriate multiplying numerical prefixes and locants, where required.
When there is a choice, low locants are assigned first to both cationic centers regardless of type (‘ium’ or ‘ylium’), and then to the ‘ylium’ centers.
Examples:
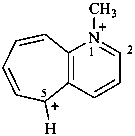
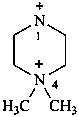
4,4-dimethylpiperazin-4-ium-1-ylium (PIN)
P-73.5.3.1 Cationic compounds with cationic centers both in the parent hydride part of the structure and on a characteristic group expressed as a cationic suffix are named by citing both cationic centers. The cationic center in the parent hydride is cited first followed by the cationic suffix.
Examples:
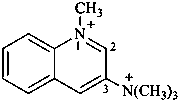
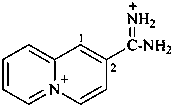
5λ5-quinolizin-5-ylium-2-carboximidamidium (PIN)
Example:
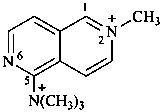 | not | 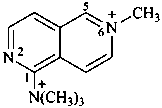 |
N,N,N,2-tetramethyl-2,6-naphthyridin-2-ium-5-aminium (PIN) (not N,N,N,6-tetramethyl-2,6-naphthyridin-6-ium-1-aminium) | ||
A polycation in which all cationic centers cannot be included in the cationic parent hydride or cationic parent compound is named by selecting one part of the structure as the parent cation and citing the other part(s) as cationic substituent prefixes. The selection of the parent cation is achieved by using the criteria for selecting the cationic parent structure. In zwitterions and in radical cations, the cationic part is always substituted into the anionic portion or into the part including a radical, in accordance with the seniority of anions and radicals over cations.
Two methods are used to name substituent structural units containing cationic centers:
(1) all prefix names are formed by adding the suffixes ‘yl’, ‘ylidene’, etc. to the cation name, preceded by the multiplying prefixes ‘di’, ‘tri’, etc. to indicate multiplicity and appropriate locants, where required. Where there is a choice for numbering, free valences receive lowest possible locants, the suffix ‘yl’ being senior to ‘ylidene’;Method (1) leads to preferred IUPAC names.(2) prefixes for expressing a monovalent substituent derived from a mononuclear parent cation denoted by ‘ium’ or by ‘onium’ described in Table 7.3 are formed by changing the ‘onium’ ending of the parent cation to ‘io’ or ‘onio’.
Examples:
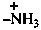
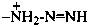
triaz-2-en-1-ium-1-yl (preselected prefix)
triaz-2-en-1-io
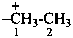
ethan-1-ium-1-yl (preferred prefix)
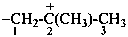
2-methylpropan-2-ylium-1-yl (preferred prefix)
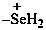
selaniumyl (preselected prefix)
selenonio
selenoniumyl
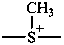
methylsulfaniumdiyl (preferred prefix)
methylsulfoniumdiyl
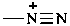
diazyn-1-ium-1-yl (preselected prefix)
diazonio
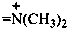
N-methylmethanaminiumylidene (preferred prefix)
(not dimethylammoniumylidene)
(not dimethylimmonio)
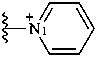
pyridin-1-ium-1-yl (preferred prefix)
pyridinio
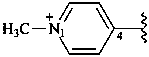
1-methylpyridin-1-ium-4-yl (preferred prefix)
A parent cationic structure is chosen by applying the following criteria in order until a definitive choice is achieved:
(a) parent with the maximum number of cationic centers, including cationic suffixes;
Example:
Example:
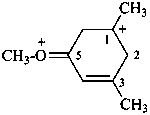
The seniority order for cations is now the order of seniority of classes rather than the order of skeletal replacement (‘a’) prefixes as used in RC-82.5.8.4, ref. 3.
Examples:
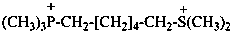
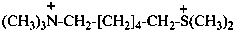
6-(dimethylsulfaniumyl)-N,N,N-trimethylhexan-1-aminium (PIN)
{not dimethyl[6-(trimethylazaniumyl)hexyl]sulfanium; N> S}
Examples:
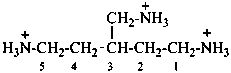
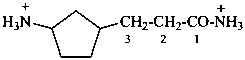
3-(3-azaniumylcyclopentyl)propanamidium (PIN)
(amidium is senior to aminium)
P-73.8.1 The suffix ‘-ylium’ is preferred to the suffix ‘-ium’ added to a parent hydride that has been modified by the λ-convention.
Example:
Examples:
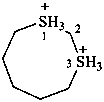
1λ4,3λ4-dithiocane-1,3-diium (PIN)
1λ6,3λ6-dithiocane-1,3-bis(ylium)
(not 1λ6,3λ4-dithiocan-3-ium-1-ylium;
identical groups, if possible, must not be named differently within a name)
P-74.0 INTRODUCTION
Zwitterionic compounds have both positive and negative ionic centers. Most examples are essentially neutral because they have an equal number of formal unit charges of opposite sign and are best illustrated by the ionic forms of amino acids. The structures in this section are all represented as zwitterionic even though some can be drawn as neutral or ionic structures.
This section also includes inner salts and dipolar compounds. Section P-74.1 will cover zwitterionic compounds with the ionic centers on the same parent compound and with ionic centers on different parent structures. Section P-74.2 deals with 1,2- and 1,3-dipolar compounds.
According to the seniority of classes, an anionic center has priority over a cationic center in zwitterions. Thus, in zwitterionic compounds anionic centers are preferred for lower locants and become the parent structure, into which the cationic part is substituted. CAS gives cationic centers priority over anionic centers.
P-74.1 ZWITTERIONIC PARENT STRUCTURES HAVING THE ANIONIC AND CATIONIC CENTERS ON THE SAME PARENT COMPOUND INCLUDING IONIC CENTERS ON CHARACTERISTIC GROUPS EXPRESSIBLE AS SUFFIXES.
P-74.1.1 Ionic centers in the same parent structure
Zwitterionic compounds with the ionic centers in the same parent structure may be named by combining appropriate cumulative suffixes at the end of the name of a parent hydride in the order ‘ium’, ‘ylium’, ‘ide’, ‘uide’. This method is preferred to the one using ionic replacement prefixes, as indicated in Sections P-72.4 and P-73.4. In either case anionic suffixes are cited after cationic suffixes in the name, and are given seniority for low locants. The final letter ‘e’ of the name of a parent hydride, or of an ‘ide’ or ‘uide’ suffix, is elided before the letter ‘i’ or ‘y’, or before a cumulative suffix beginning with a vowel. Multiplying prefixes ‘di-’, ‘tri-’, etc., or ‘bis-’, ‘tris-’, etc. as appropriate for each type of suffix, are added to specify the number of each kind of ionic center. Where there is a choice, lowest locants are given to the ionic centers in the following order, listed in decreasing order of seniority: ‘uide’ (‘uida’), ‘ide’ (‘ida’), ‘ylium’ (‘ylia’), and ‘ium’ (‘onia’).
For nomenclature purposes, zwitterionic compounds having the ionic centers in the same parent structure are not considered as neutral compounds. This is a change in the methodology described in RC-84.1.1 (ref. 3) and is illustrated in the last example below.
Examples:
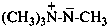
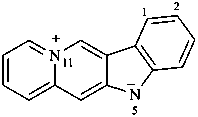
5H-11λ5-indolo[2,3-b]quinolizin-11-ylium-5-ide (PIN)
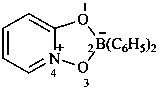
2,2-diphenyl-4λ5-[1,3,4,2]dioxazaborolo[4,5-a]pyridin-4-ylium-2-uide (PIN)
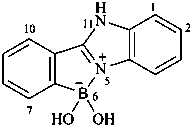
6,6-dihydroxy-6,11-dihydro-5λ5-[1,3]benzimidazolo[1,2-b][2,1]benzazaborol-5-ylium-6-uide (PIN) 6,6-dihydroxy-6,11-dihydro-5λ5-benzimidazolo[1,2-b][2,1]benzazaborol-5-ylium-6-uide
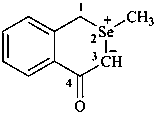
2-methyl-4-oxo-3,4-dihydro-1H-2-benzoselenopyran-2-ium-3-ide (PIN)
(not 2-methyl-3,4-dihydro-1H-2-benzoselenopyran-2-ium-3-id-4-one)
2-methyl-4-oxo-3,4-dihydro-1H-isoselenochromen-2-ium-3-ide
(not 2-methyl-3,4-dihydro-1H-isoselenochromen-2-ium-3-id-4-one)
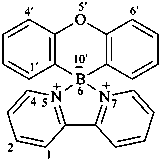
5λ5,7λ5-spiro[[1,3,2]diazaborolo[3,4-a:5,1-a′]dipyridine-6,10′-phenoxaborinine]-5,7-bis(ylium)-6-uide (PIN)
P-74.1.2 Zwitterionic compounds with at least one ionic center on a characteristic group
Zwitterionic compounds with at least one ionic center on a characteristic group may be named by adding the appropriate ionic suffix to the name of the ionic parent hydride. In names, cationic suffixes are cited before anionic suffixes. For assignment of lower locants, ionic centers on skeletal atoms of the parent hydride are preferred to the locants for positions of attachment of characteristic groups denoted by ionic suffixes.
Examples:
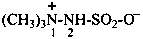
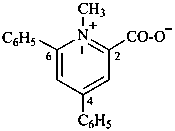
1-methyl-4,6-diphenylpyridin-1-ium-2-carboxylate (PIN)
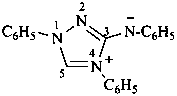
N,1,4-triphenyl-1H-1,2,4-triazol-4-ium-3-aminide (PIN)
N-(1,4-diphenyl-1H-1,2,4-triazol-4-ium-3-yl)benzenaminide
Zwitterionic compounds with anionic and cationic centers on different parent structures may be named by prefixing the name of the cationic center or the parts of the structure containing the cationic centers to the name of the anionic parent structure.
Examples:
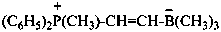
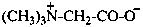
(N,N-dimethylmethanaminiumyl)acetate (PIN)
(trimethylammoniumyl)acetate
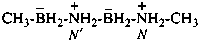
methyl{[(methanaminiumyl)boranuidyl]azaniumyl}boranuide (PIN)
2,4-diaza-3,5-diborahexane-2,4-diium-
3,5-diuide 3,5-diazonia-2,4-diboranuidahexane
[not 1-methyl-3-(methanaminiumyl)diborazan-2-ium-1,3-diuide;
names such as diborazane are no longer named as parent hydrides, see P-21.2.3.1)
Dipolar compounds are electrically neutral molecules carrying a negative and a positive charge in at least one of their major canonical resonance structures. In most dipolar compounds the charges are delocalized; however the term is also applied to species where this is not the case. 1,2-Dipolar compounds have the opposite charges on adjacent atoms. The term 1,3-dipolar compounds is used for those in which a significant canonical resonance form can be represented by a separation of charge over three atoms.
P-74.2.1 1,2-Dipolar compoundsP-74.2.1 1,2-Dipolar compounds
P-74.2.2 1,3-Dipolar compounds
P-74.2.3 Dipolar substituent groups
P-74.2.1.1 ‘Ylides’
Compounds in which an anionic site ‘Y–’ (originally only on carbon, but now including other atoms) is attached directly to a heteroatom ‘X+’ (usually nitrogen, phosphorus, sulfur, selenium, or tellurium) carrying a formal positive charge are 1,2-dipolar species of the type RmX+-Y––Rn. If ‘X’ is a saturated atom of an element from the second row of the periodic system, the ‘ylide’ is commonly represented by a charge-separated form; if ‘X’ is a third, fourth, etc. row element uncharged canonical forms are usually shown, RmX=YRn.
These ‘ylides’ are subdivided into subclasses: nitrogen ylides, phosphorus ylides, oxygen ylides, sulfur ylides, etc. They may be named in different ways depending on the nature of the atoms ‘X’ and ‘Y’:
(1) as zwitterionic compounds;Method (1) is applicable to all ‘ylides’ and leads to preferred IUPAC names.(2) by applying the λ-convention when X = P, As, Sb, Bi, S, Se or Te;
(3) by functional class nomenclature using the class names oxide, sulfide, imides.
P-74.2.1.1.1 Nitrogen ylides
Nitrogen ylides have the general structure R3N+–C–R2.
Example:
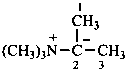
Phosphorus ylides have the general structure R3P+–C–R2 ↔ R3P=CR2
Example:
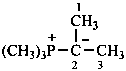
Oxygen ylides have the general structure R2O+–C–R2.
Example:
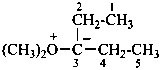
Sulfur ylides have the general structure R2S+–C–R2 ↔ R2S=CR2.
Example:
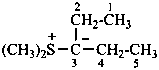
P-74.2.1.2 Amine oxides, imine oxides, and their chalcogen analogues
Amine oxides and imine oxides have the generic formulae R3N+–O– and R2C=N+(R)–O– respectively; chalcogen analogues are amine sulfides, imine selenides, etc. (where O is replaced by S, Se, or Te). They may be named:
(1) as zwitterionic compounds;Method (2) leads to preferred IUPAC names when one amine oxide is present. When two amine oxides are present, the λ-convention is used to generate PINs (see P-62.5). Hence, zwitterionic compounds are never PINs (see P-62.5).(2) by functional class nomenclature using the functional class name ‘oxide’, sulfide, ‘selenide’, or ‘telluride’.
Example:
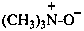
Amine imides (not amine imines) have the generic formula R3N+–N––R. They may be named by two methods:
(1) as a zwitterion based on hydrazine (in order not to break the nitrogen chain);Method (1) leads to preferred IUPAC names.(2) by functional class nomenclature using the class name ‘imide’ placed after the name of the amine.
Example:
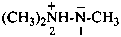
Phosphine oxides have the generic formula R3P+–O– ↔ R3P=O. Chalcogen analogues are phosphine sulfides, phosphine selenides, and phosphine telluride (where O is replaced by S, Se, and Te, respectively). They may be named by three methods:
(1) as zwitterionic compounds;Method (3) leads to preferred IUPAC names.(2) by functional class nomenclature using the class names oxide, sulfide, selenide, or telluride;
(3) substitutively, as heterones, by using the suffix ‘-one’ and λ5-phosphane as the parent hydride.
Example:
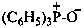
P-74.2.1.5 Phosphine imides
Phosphine imides have the generic structure: R3P+–N––R ↔ R3P=N–R. They may be named in three ways:
(1) as zwitterionic compounds;Method (3) leads to preferred IUPAC names.(2) by functional class nomenclature using the class name imide;
(3) substitutively, as heterimines, by using the suffix ‘-imine’ and λ5-phosphane as the parent hydride.
Example:
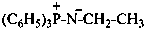
P-74.2.2 1,3-Dipolar compounds
The term 1,3-dipolar compounds is used for those compounds in which a significant canonical resonance can be represented by a distribution of charge over three atoms. The subclasses of 1,3-dipolar compounds include:
P-74.2.2.1 the allyl (propenyl) typeP-74.2.2.1 Allyl type compounds have the following delocalized general structure where Y and/or Z = C, N, or O; and X = N or O:
P-74.2.2.2 the propargyl (prop-2-yn-1-yl) type
P-74.2.2.3 the carbene type
(1) by using a parent hydride to generate a zwitterion;Preferred IUPAC names are those expressing the zwitterionic nature of the compounds. Three exceptions are recognized:(2) by substituting a cationic substituent into a parent anion;
(3) by functional class nomenclature using the class names imide, oxide, etc.;
(4) by using the λ-convention.
(1) heteroatom oxides as described in P-74.2.2.1.4 for azoxy compounds and as described in P-74.2.2.1.9 for nitrones;P-74.2.2.1.1 Azo imides, analogous to azoxy compounds, have the following delocalized general structure:(2) use of the λ-convention as in P-74.2.2.1.8 for heterone S-oxides.
Example:
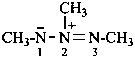
Example:
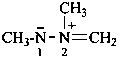
Example:
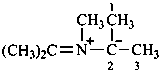
Example:
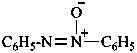
Example:
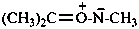
Example:
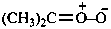
Example:
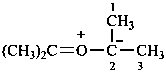
Examples:
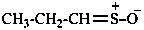
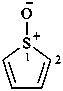
1H-1λ4-thiophen-1-one (PIN)
thiophene oxide
(thiophen-1-ium-1-yl)oxidanide
1-oxo-1H-1λ4-thiophene
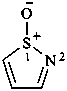
1H-1λ4,2-thiazol-1-one (PIN)
1,2-thiazole 1-oxide
1,2-thiazole S-oxide
Example:
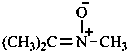
Example:
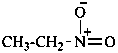 | ↔ CH3-CH2-NO2 |
| nitroethane (PIN) | |
P-74.2.2.2 The propargyl (propynyl) type includes compounds having the following canonical resonance forms:
(1) as zwitterionic compounds, without breaking the longest chain of heteroatoms;P-74.2.2.2.1.1 Nitrile imides.(2) by functional class nomenclature using the class names imide, oxide, sulfide, etc.
Method (1) in P-74.2.2.2.1, zwitterionic names, generates preferred IUPAC names.
Example:
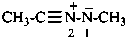
Method (2) in P-74.2.2.2.1, functional class names, yields preferred IUPAC names (see also P-66.5.4.1).
Examples:
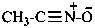
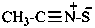
acetonitrile sulfide (PIN)
(ethylidyneazaniumyl)sulfanide
Method (1) in P-74.2.2.2.1, zwitterionic names, gives preferred IUPAC names.
Example:
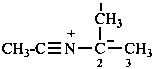
(1) substitutively, using the compulsory prefix azido (P-61.7);Method (1) yields preferred IUPAC names (see also P-61.7)(2) by functional class nomenclature using the class name azide;
(3) as derivatives of the zwitterionic parent hydride ‘triazadien-2-ium-1-ide.’
Example:
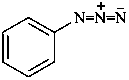
(1) substitutively by using the compulsory prefix diazo (P-61.4);.Method (1) leads to preferred IUPAC names (see also P-61.4).(2) as derivatives of the zwitterionic parent hydride diazen-2-ium-1-ide.
Example:
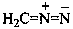
Example:
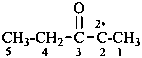
Compounds having the structure RC(=NH)-C2•–R are imidoyl carbenes. Imidoyl is a shortened, but imprecise, term for carboximidoyl, RC(=NH)–. These carbenes may be named by two methods:
(1) by using the longest carbon chain, according to the principles of substitutive nomenclature for radicals, low locants being assigned to the radical to be cited as suffix;Method (1) leads to preferred IUPAC names.(2) substitutively on the basis of the appropriate carbene as the parent structure.
Example:
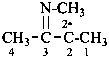
Example:
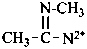
Preferred IUPAC names are formed by using the longest carbon chain according to the principles of substitutive nomenclature for radicals, low locants being assigned to the suffix ‘ylidene’.
Example:
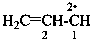
Names of dipolar substituent groups are formed by using prefixes for naming ions as the substituent group and designating the free valences by the suffixes ‘yl’, ‘ylidene’ or ‘ylidyne’.
Examples:
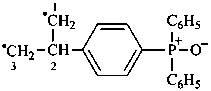
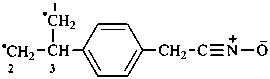
2-{4-[2-(oxidoazaniumylidyne)ethyl]phenyl}propane-1,3-diyl (PIN)
2-{4-[2-(oxidoammoniumylidyne)ethyl]phenyl}propane-1,3-diyl
For the purpose of nomenclature of organic chemistry, a radical ion is a molecular entity having at least one radical center and one ionic center, which may be on the same or on different atoms of a parent structure. They are formally named as described in the following subsections.
P-75.1 Radical ions formed by the addition or removal of electronsP-75.1 RADICAL IONS FORMED BY THE ADDITION OR REMOVAL OF ELECTRONS
P-75.2 Radical ions derived from parent hydrides
P-75.3 Radical ions on characteristic groups
P-75.4 Ionic and radical centers in different parent structures
Radical ions formed by the addition or removal of electrons may be named in two ways.
(1) by using the suffixes ‘elide’ and ‘elium’ in substitutive nomenclature, whereby radical ions derived formally from a neutral parent hydride, parent compound, or hydro derivative of either by the addition or removal of electrons may be named by adding the suffixes ‘elide’ or ‘elium’ to the name of the neutral parent structure, the number of added or removed electrons is denoted by numerical prefixes, ‘di’, ‘tri’, etc.The substitutive method (1) leads to preferred IUPAC names.Note: This new method may be used to indicate a global structure, when the positions of the radical and/or ionic centers are not known, or when it is not necessary, nor desirable, to name a specific structure. These suffixes cannot be used in the presence of other suffixes.
(2) by functional class nomenclature, whereby radical ions derived formally from a neutral parent hydride, parent compound, or hydro derivative of either by the addition or removal of electrons may be named by adding the terms ‘radical cation’ or ‘radical anion’ as separate words to the name of the neutral parent hydride or parent compound having the same molecular formula; the multiplying prefixes ‘di’, ‘tri’ etc. are used to denote multiple radical or ionic centers; the terms ‘radical ion’ can also be used, followed by a charge number indicating the appropriate charge sign.
Examples:
[C10H8]•+
azulenelium (PIN)
azulene radical cation
azulene radical ion(1+)
The order of seniority, radicals > anions > cations, is reflected in a preferred IUPAC name. Suffixes assigned to anionic and/or cationic centers are placed first after the name of the parent structure (parent hydride, functional parent hydride, or functionalized parent hydride), followed by suffixes attributed to radical centers.
A radical ion derived formally by the removal of one or more hydrogen atoms from a single skeleton atom or from different skeletal atoms of an ionic or zwitterionic parent hydride is named by adding to its name the suffixes ‘yl’ or ‘ylidene’ with appropriate multiplying prefixes before ‘yl’ or ‘ylidene’, with elision of the final letter ‘e’ of the name of the ionic parent hydride. Skeletal positions with radical centers have preference over those with ionic centers for assignment of low locants.
P-75.2.1 Examples of radical anions:
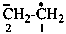
ethan-2-id-1-yl (PIN)
(CH3)3B•–
trimethylboranuidyl (PIN)
trimethyl-1λ5-boranidyl
1λ4-thiiran-1-id-1-yl (PIN)
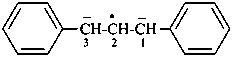
1,3-diphenylpropane-1,3-diid-2-yl (PIN)
CH3-O-CO-C2•–
(methoxycarbonyl)methanidylidene (PIN)
H4Si•+
silaniumyl (preselected name)
[CH3-CH2]•2+
ethaniumyliumyl (PIN)
(location of radical and ionic centers is unknown)
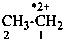
ethan-1-ium-1-ylium-1-yl (PIN)
(location of radical and ionic centers as indicated by locants)
 | or [C6H6]•+ |
| benzenelium (PIN) benzeniumyl |
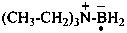
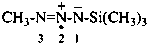
3-methyl-1-(trimethylsilyl)triaz-2-en-2-ium-1-id-2-yl (PIN)
Radical and ionic centers at positions in a mancude parent hydride where there is an insufficient number of hydrogen atoms to directly apply recommendations for the use of ‘yl’, ‘ylidene’, ‘ide’ or ‘ylium’ as given in P-71.1, P-72.1 and P-73.2, respectively are derived formally from a dihydro derivative of the cyclic parent hydride. Radical ions can also be described by applying the principle of ‘added indicated hydrogen’ (see P-14.7 and P-58.2.2). In this method, the ‘hydro’ derivative is described by specifying the hydrogen atom of a dihydro pair that remains after the radical center is created by citing in italic capital H and the locant of the skeletal atom to which the hydrogen atom resides, both enclosed in a set of parentheses and inserted into the name of the corresponding parent hydride immediately after the locant for the radical center. The ionic center is created next, by subtraction of a hydron. For clarity of names, the ‘added hydrogen’ is cited in names. Preferred IUPAC names are formed by the ‘added indicated hydrogen’ method (see P-58.2).
Examples:
1,4-dihydronaphthalen-4-id-1-yl
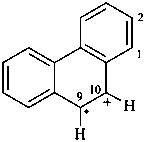
9,10-dihydrophenanthren-10-ylium-9-yl (PIN)
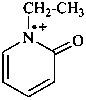
1-ethyl-2-oxopyridin-1-ium-1(2H)-yl (PIN)
1-ethyl-2-oxo-1,2-dihydropyridin-1-ium-1-yl
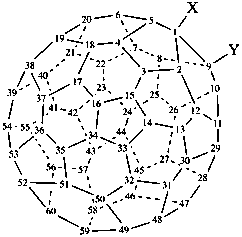
X = • ; Y = +
(C60-Ih)[5,6]fulleren-9-ylium-1(9H)-yl (PIN)
1,9-dihydro(C60-Ih)[5,6]fulleren-9-ylium-1-yl
X = • ; Y = –
C60-Ih)[5,6]fulleren-9-id-1(9H)-yl (PIN)
1,9-dihydro(C60-Ih)[5,6]fulleren-9-id-1-yl
P-75.3.1 Radical ions on ionic suffix groups
When ions may be named by using modified suffixes (see P-73.1.2.1 and P-72.2.2.2.3), the suffixes denoting radical centers are added to the name of the cationic or anionic parent hydride.
Examples:
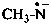
methanaminidyl (PIN)
CH3-CO-N•+
acetamidyliumyl(PIN)
C6H5-C≡N•+
benzonitriliumyl (PIN)
Examples:
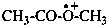
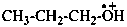
propyloxidaniumyl (PIN)
CH3-CO-N•–
acetylazanidyl (PIN)
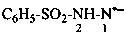
2-(benzenesulfonyl)hydrazin-1-id-1-yl (PIN)
2-(benzenesulfonyl)diazan-1-id-1-yl
Radical centers have priority over ionic centers. A radical ion derived formally by the subtraction of one or more hydrogen atom(s) from an ionic or zwitterionic compound in which the ionic and radical centers cannot be included in the same parent structure is named by expressing the ionic center(s), or the part of the structure containing the ionic center(s), by means of substituent prefixes attached to the name of the parent radical.
Examples:
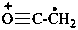
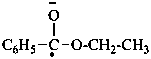
ethoxy(oxido)(phenyl)methyl (PIN)
α-ethoxy-α-oxidobenzyl
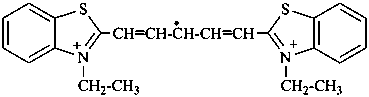
1,5-bis(3-ethyl-1,3-benzothiazol-3-ium-2-yl)penta-1,4-dien-3-yl (PIN)
Delocalization in names involving one radical or ionic center in an otherwise conjugated double bonds structure is denoted by the appropriate suffix without locants.
Examples:
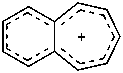
benzo[7]annulenylium (PIN)
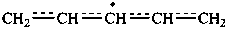
pentadienyl (PIN)
P-77.1 PREFERRED NAMES FOR SALTS OF ORGANIC BASES
P-77.1.1 Preferred IUPAC names for salts of organic bases are binary names formed by citing the name of the cation followed by that of the anion.
Examples:
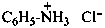
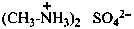
bis(methanaminium) sulfate (PIN)
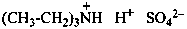
N,N-diethylethanaminium hydrogen sulfate (PIN)
Example:
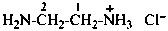
(1) as adducts. The names of these adducts are preferred IUPAC names only when the acid components of the adducts are organic compounds;Example:(2) the unaltered name of the base followed by the name of the anion;
(3) for salts of hydrohalogen acids only, the unaltered name of the base is followed by hydrofluoride, hydrobromide, hydrochloride, or hydroiodide, as the case may be.
P-77.2.1 Preferred IUPAC names are binary names formed by citing the name of the cation followed by that of the anion (see P-72.2.2.2.2).
Examples:
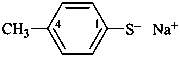
sodium 4-methylbenzene-1-thiolate (PIN)
Example:
P-77.3.1 Preferred IUPAC names are binary names formed by citing the name of the cation followed by that of the anion (see P-72.2.2.2.1).
Example:
Example:
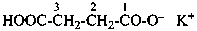
Return to:
Blue Book home page.